Question
Part F. Molecular Formulas and Empirical Formulas 11. Compute the empirical and molecular formulas for the following elemental analysis. H. Molecular CI Weight 32.0%
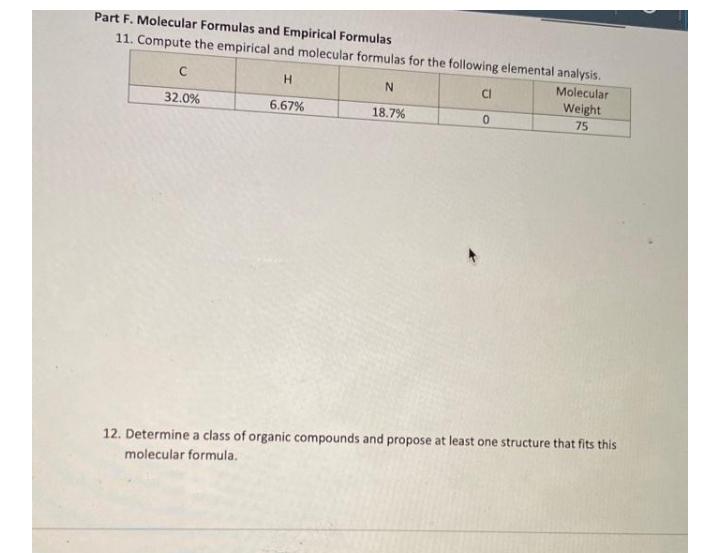
Part F. Molecular Formulas and Empirical Formulas 11. Compute the empirical and molecular formulas for the following elemental analysis. H. Molecular CI Weight 32.0% 6.67% 18.7% 75 12. Determine a class of organic compounds and propose at least one structure that fits this molecular formula.
Step by Step Solution
3.57 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Molecular and Empirical Formula are the same C2H5NO2To find ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Investment Analysis and Portfolio Management
Authors: Frank K. Reilly, Keith C. Brown
10th Edition
538482109, 1133711774, 538482389, 9780538482103, 9781133711773, 978-0538482387
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



