Answered step by step
Verified Expert Solution
Question
1 Approved Answer
plzzz answer the missing parts and show detailed stepss!! can you also chevk that my work is correct? thank you so much! i have attached
plzzz answer the missing parts and show detailed stepss!! can you also chevk that my work is correct? 

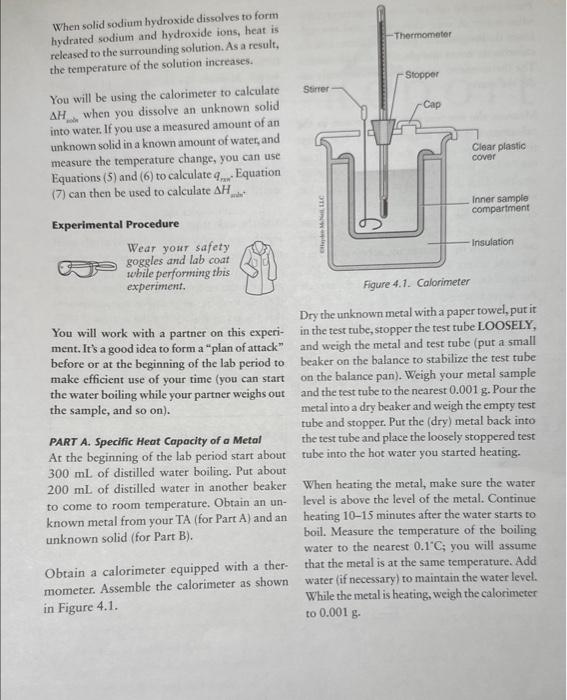

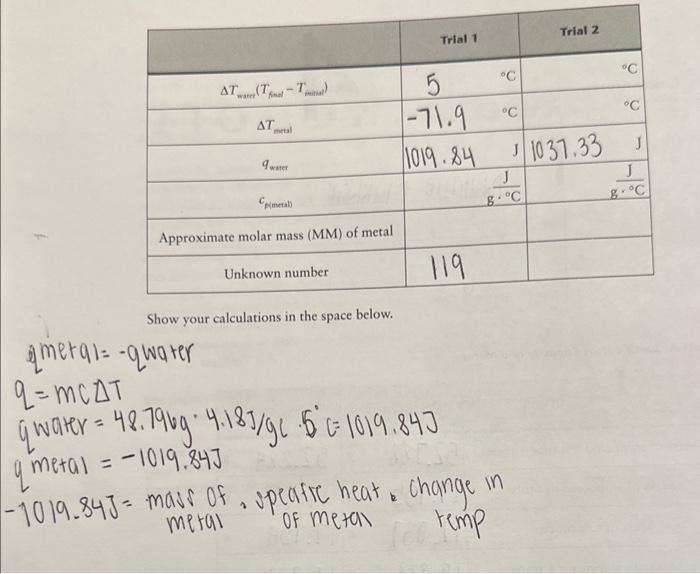
of a Metal Show your calculations in the space below. qmetal=qwaterq=mcTqwater=48.79g4.18g/gc6c=1019.84Jgmetal=1019.84J PART B. Heat of Solution .745.5.820g17.0925g=48.72755.159g0.014g=5.019g48.7275g4.18J/gg.9.9fc=2.12rn=1201.72J5.014g/101.11g/mol=0.05mo1201.72J/0.05mol=24034J/mo Show your calculations in the space below, use tne nex pagem mmary. Calorimeters are devices used to measure the heat absorbed or evolved during a process. A calorimeter (which can be something as simple as a styrofoam cup) has insulating walls to prevent heat exchange between the contents of the calorimeter and its surroundings. You will be using a simple calorimeter to calculate the specific heat capacity of a metal. The specific heat capacity, often simply called specific heat, is the quantity of heat required to raise the temperature of one gram of a given substance by 1C (or 1K ) at constant pressure. In the following equation, specific heat capacity (cp, where the subscript p indicates constant pressure) is used as a proportionality constant and allows us to calculate the quantity of heat (q) that must be added to effect a temperature change (T=TfinalTintial) to a certain substance with mass m q=cpmT The specific heat capacities for some common substances are listed below: Amounts of heat are measured in joules (J). To raise the temperature of 1g of water by 1C,4.18 joules of heat are required. Therefore, the specific heat capacity of water is 4.18J/(gC). (Since 1 calorie = 4.18 joules, we could also say that the specific heat capacity of water is 1cal/(gC).) To determine the specific heat capacity of a In the second part of the experiment, we will metal, a weighed amount of metal is heated to measure the energy associated with dissolving some known temperature and is then placed into an ionic substance in water. In this specific case a calorimeter that contains a measured amount we refer to the energy change as the enithalpy of of water at a known temperature. Hear will flow solution (Hund) or heat of solution. from the metal to the water and, given sufficient rime, both will reach the same temperature. The If an aqueous reaction takes place in a calorim. heat lost by the metal is equal to the amount of eter, heat will either be released or absorbed by heat absorbed by the water (assuming that no the solution contained within the calorimeter. heat is absorbed by the calorimeter walls and no Again, we are simplifying things by assuming heat is lost from the calorimeter to its surround- that no heat is absorbed by the calorimeter walls ings). This is expressed thermodynamically as: or lost to the surroundings. If the temperature qmial=qmart (2) been given off by the reaction (qos is negative, Combining equations (1) and (2) gives: exothermic). If the temperature of the solution decreases, then heat must have been absorbed by the reaction (3) ( qmin is positive, endothermic). Similar to Equation (2), the heat change of the reaction is equal Dulong and Petit discovered a simple relation- in magnitude but opposite in sign to the heat ship between specific heat capacity and molar change associated with the solution. mass for metallic elements. The relationship is: MM=25/cp (4) qmen=qiounca Changes in energy always accompany chemical Filute aqueous solutions we assume that the: reactions. As a reaction occurs, energy is either of water and solution is entirely due to the mass transferred to or is absorbed from the surround. the solution is equat the specific heat capacity of ings. The energy transferred during the reaction. is in the form of work andlor heat. In addition to measuring the energy exchanged between a piece of hot metal and a sample of water, a Because the reaction takes place at constant calorimeter may be used to determine the energy pressure, the heat released or absorbed by the released or absorbed during a chemical reac- reaction is also equal to the enthalpy change: tion. This quantity is known as the enthalpy of reaction (Hm=n), also referred to as the heat of reaction. Hnon is normally expressed in terms of units of energy per mole-typically J/mol. An example of an exothermic reaction is the following: NaOH When solid sodium hydroxide dissolves to form. hydrated sodium and hydroxide ions, heat is released to the surrounding solution. As a result, the temperature of the solution increases. You will be using the calorimeter to calculate Hwin when you dissolve an unknown solid into water. If you use a measured amount of an unknown solid in a known amount of water, and measure the temperature change, you can use Equations (5) and ( 6 ) to calculate qmm.. Equation (7) can then be used to calculate Hmen Experimental Procedure Wear your safety goggles and lab coat while performing this experiment. Dry the unknown metal with a paper towel, put it You will work with a partner on this experi- in the test tube, stopper the test tube LOOSELY, ment. It's a good idea to form a "plan of attack" and weigh the metal and test tube (put a small before or at the beginning of the lab period to beaker on the balance to stabilize the test tube make efficient use of your time (you can start on the balance pan). Weigh your metal sample the water boiling while your partner weighs out and the test tube to the nearest 0.001g. Pour the the sample, and so on). metal into a dry beaker and weigh the empry test tube and stopper. Put the (dry) metal back into At the beginning of the lab period start about tube into the hot water you started heating. 300mL of distilled water boiling. Put about 200mL of distilled water in another beaker When heating the metal, make sure the water to come to room temperature. Obtain an un- level is above the level of the metal. Continue known metal from your TA (for Part A) and an heating 10-15 minutes after the water starts to unknown solid (for Part B). boil. Measure the temperature of the boiling water to the nearest 0.1C; you will assume Obtain a calorimeter equipped with a ther- that the metal is at the same temperature. Add mometer. Assemble the calorimeter as shown water (if necessary) to maintain the water level. in Figure 4.1. While the metal is heating, weigh the calorimeter to 0.001g. Place about 50mL of room temperature, dis- PART B. Heat of Solution tilled water in your calorimeter and reweigh. Weigh your dry calorimeter to the nearest Insert the thermometer probe and stirrer into the 0.001g. Place about 50mL of room temperacalorimeter cover and place on the calorimeter. ture, distilled water in your calorimeter and reThe thermometer tip should be completely un- weigh. Measure the temperature of the water der the water. inside the calorimeter (to the nearest 0.1C ). Measure the temperature of the water in the Weigh the packet containing your sample, then calorimeter (to the nearest 0.1C ). Use a test reweigh after the sample has been transferred to tube holder to remove the hot test tube from determine the mass of the sample by difference. the boiling water. Quickly pour the hot metal The solids are anhydrous, so keep the packet into the water in the calorimeter. Make sure that sealed until you transfer it to the calorimeter. water adhering to the outside of the test tube does not run into the calorimeter when you are Add the unknown solid to your calorimeter. pouring the hot metal. Replace the calorimeter Stir the contents continuously (and gently swirl cover and stir the water inside as best as you can the calorimeter). Continuous, gentle swirling with the stirrer. Record (to the nearest 0.1C ) is required to ensure even heating of the water the final (minimum or maximum) temperature and an accurate temperature reading. Measure the water reaches. the final temperature (minimum or maximum) that the water reaches (to the nearest 0.1C ). experiment a second time. Make sure all of the solid dissolves. You should do not see a change of at least 5C. If you your TA when you are finished with it. Do not used (see your TA first). forget to record the unknown number! Waste Disposal: Dispose of the solution in Part B in the waste container thank you so much! 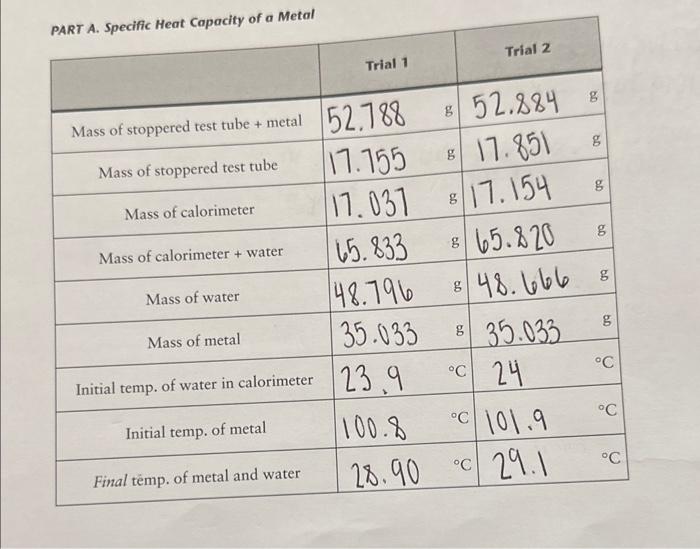
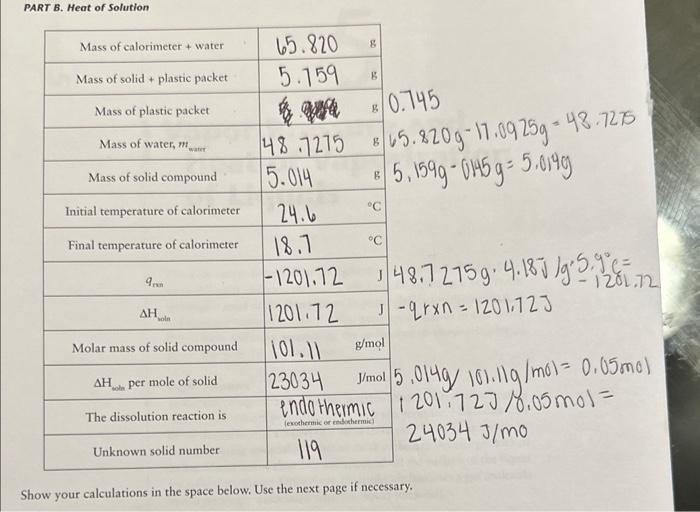



i have attached the lab report to assist you




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


