Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(PO2, CO2, C4) a) Despite the main reaction in the partial oxidation of propylene (C3H6) to give acrylic acid (C3H4O2), there are two other
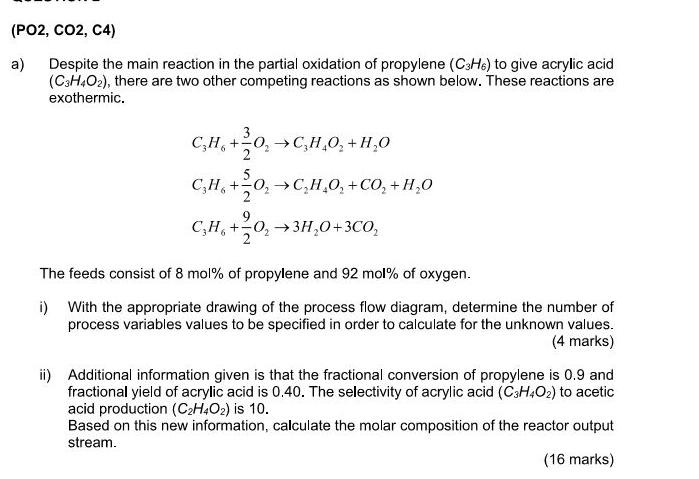
(PO2, CO2, C4) a) Despite the main reaction in the partial oxidation of propylene (C3H6) to give acrylic acid (C3H4O2), there are two other competing reactions as shown below. These reactions are exothermic. C3H6 + +0C,HO+HO 5 CHOCHO + CO+HO CH+03HO+3CO The feeds consist of 8 mol% of propylene and 92 mol% of oxygen. i) With the appropriate drawing of the process flow diagram, determine the number of process variables values to be specified in order to calculate for the unknown values. (4 marks) ii) Additional information given is that the fractional conversion of propylene is 0.9 and fractional yield of acrylic acid is 0.40. The selectivity of acrylic acid (C3H4O2) to acetic acid production (C2H4O2) is 10. Based on this new information, calculate the molar composition of the reactor output stream. (16 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The ans...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


