Answered step by step
Verified Expert Solution
Question
1 Approved Answer
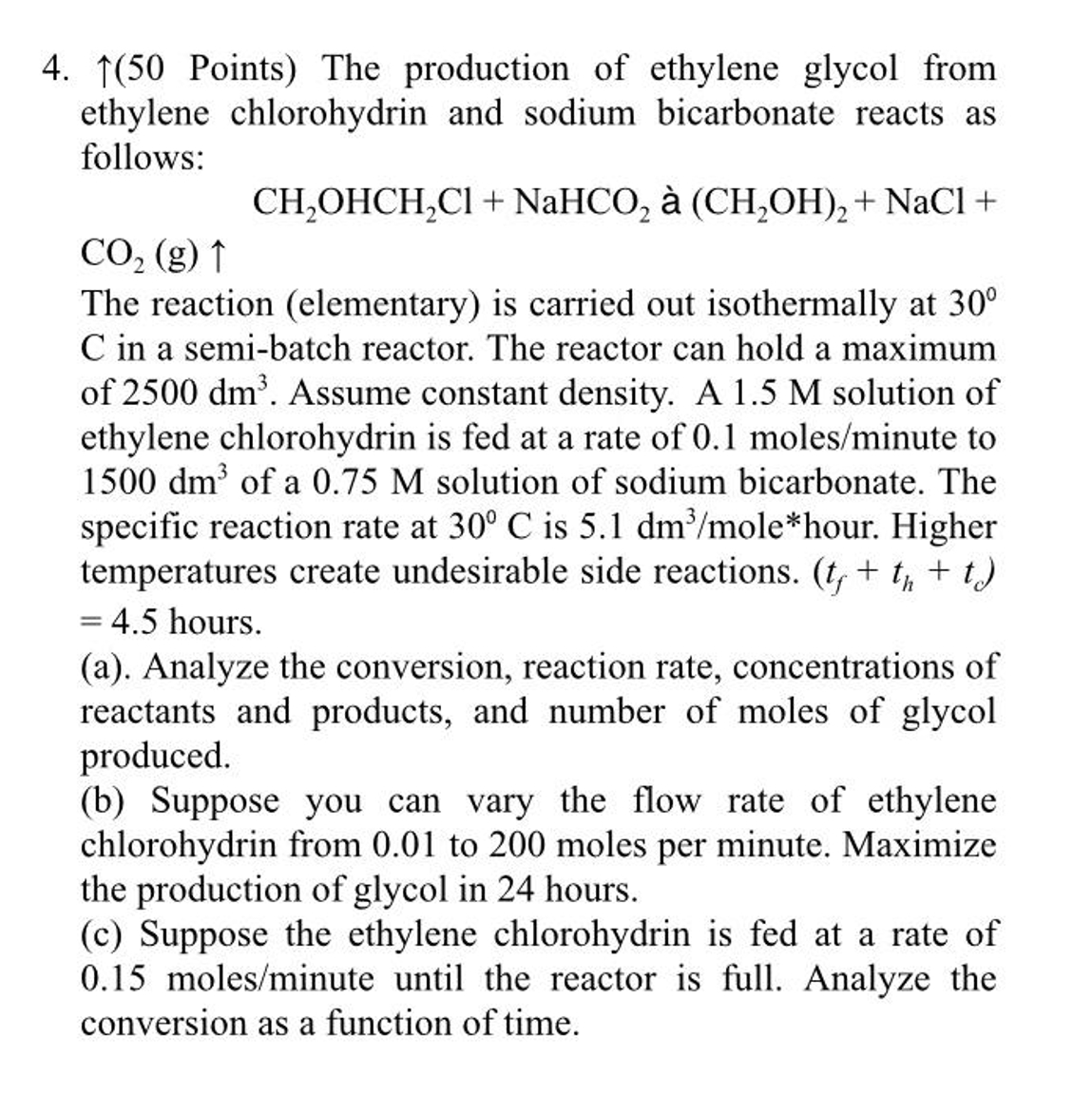
Points ) The production of ethylene glycol from ethylene chlorohydrin and sodium bicarbonate reacts as follows: CH _ ( 2 ) OHCH _ ( 2
Points The production of ethylene glycol from
ethylene chlorohydrin and sodium bicarbonate reacts as
follows: CHOHCHClNaHCOCHOHNaCl The reaction elementary is carried out isothermally at
in a semibatch reactor. The reactor can hold a maximum
of Assume constant density. A solution of
ethylene chlorohydrin is fed at a rate of molesminute to
of a solution of sodium bicarbonate. The
specific reaction rate at is molehour Higher
temperatures create undesirable side reactions.
hours.
a Analyze the conversion, reaction rate, concentrations of
reactants and products, and number of moles of glycol
produced.
b Suppose you can vary the flow rate of ethylene
chlorohydrin from to moles per minute. Maximize
the production of glycol in hours.
c Suppose the ethylene chlorohydrin is fed at a rate of
moleinute until the reactor is full. Analyze the
conversion as a function of time.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


