Question
Polyethylene oxide has a melting point of 65 C, while isotactic polystyrene has a melting point of 225 C (Figure 1d). Explain the effect
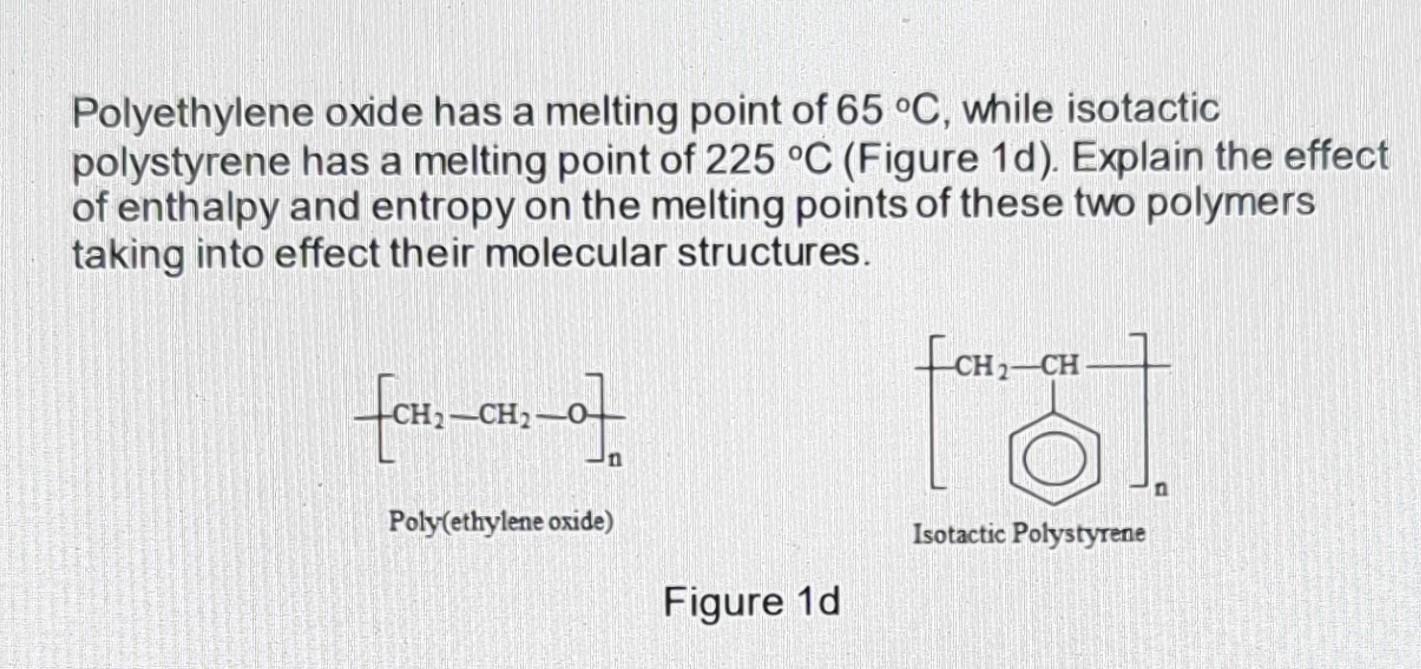
Polyethylene oxide has a melting point of 65 C, while isotactic polystyrene has a melting point of 225 C (Figure 1d). Explain the effect of enthalpy and entropy on the melting points of these two polymers taking into effect their molecular structures. [CH-CH_0] Poly(ethylene oxide) Figure 1d format CH CH Isotactic Polystyrene
Step by Step Solution
3.33 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Enthalpy He Heat Content of a system while Entopy related to the disorder or randomness of molecules ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Materials Science and Engineering An Integrated Approach
Authors: David G. Rethwisch
4th Edition
1118214226, 1118061608, 9781118214220, 978-1118061602
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



