Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Polyethylene terephtalate (Mylar, Dacron) is cooled rapidly from 300 C (state 1) to room temperature. The resulting material is rigid and perfectly transparent (state
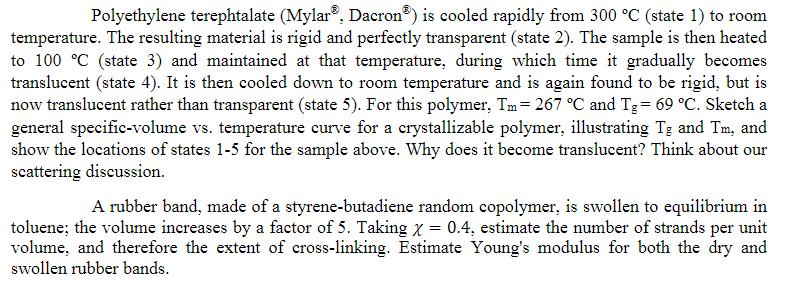
Polyethylene terephtalate (Mylar, Dacron) is cooled rapidly from 300 C (state 1) to room temperature. The resulting material is rigid and perfectly transparent (state 2). The sample is then heated to 100 C (state 3) and maintained at that temperature, during which time it gradually becomes translucent (state 4). It is then cooled down to room temperature and is again found to be rigid, but is now translucent rather than transparent (state 5). For this polymer, Tm= 267 C and Tg = 69 C. Sketch a general specific-volume vs. temperature curve for a crystallizable polymer, illustrating Tg and Tm, and show the locations of states 1-5 for the sample above. Why does it become translucent? Think about our scattering discussion. A rubber band, made of a styrene-butadiene random copolymer, is swollen to equilibrium in toluene; the volume increases by a factor of 5. Taking x = 0.4, estimate the number of strands per unit volume, and therefore the extent of cross-linking. Estimate Young's modulus for both the dry and swollen rubber bands. Polyethylene terephtalate (Mylar, Dacron) is cooled rapidly from 300 C (state 1) to room temperature. The resulting material is rigid and perfectly transparent (state 2). The sample is then heated to 100 C (state 3) and maintained at that temperature, during which time it gradually becomes translucent (state 4). It is then cooled down to room temperature and is again found to be rigid, but is now translucent rather than transparent (state 5). For this polymer, Tm= 267 C and Tg = 69 C. Sketch a general specific-volume vs. temperature curve for a crystallizable polymer, illustrating Tg and Tm, and show the locations of states 1-5 for the sample above. Why does it become translucent? Think about our scattering discussion. A rubber band, made of a styrene-butadiene random copolymer, is swollen to equilibrium in toluene; the volume increases by a factor of 5. Taking x = 0.4, estimate the number of strands per unit volume, and therefore the extent of cross-linking. Estimate Young's modulus for both the dry and swollen rubber bands.
Step by Step Solution
★★★★★
3.35 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION 1 Specificvolume vs temperature curve for a crystallizable polymer Heres a sketch of a typical specificvolume vs temperature curve for a crystallizable polymer like polyethylene terephthalate ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


