Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Polymer Engineering Find the transition temperature of the glass with the specified volume having the equation V() (L) = 1.137 + 1.4 X 10^-4 T

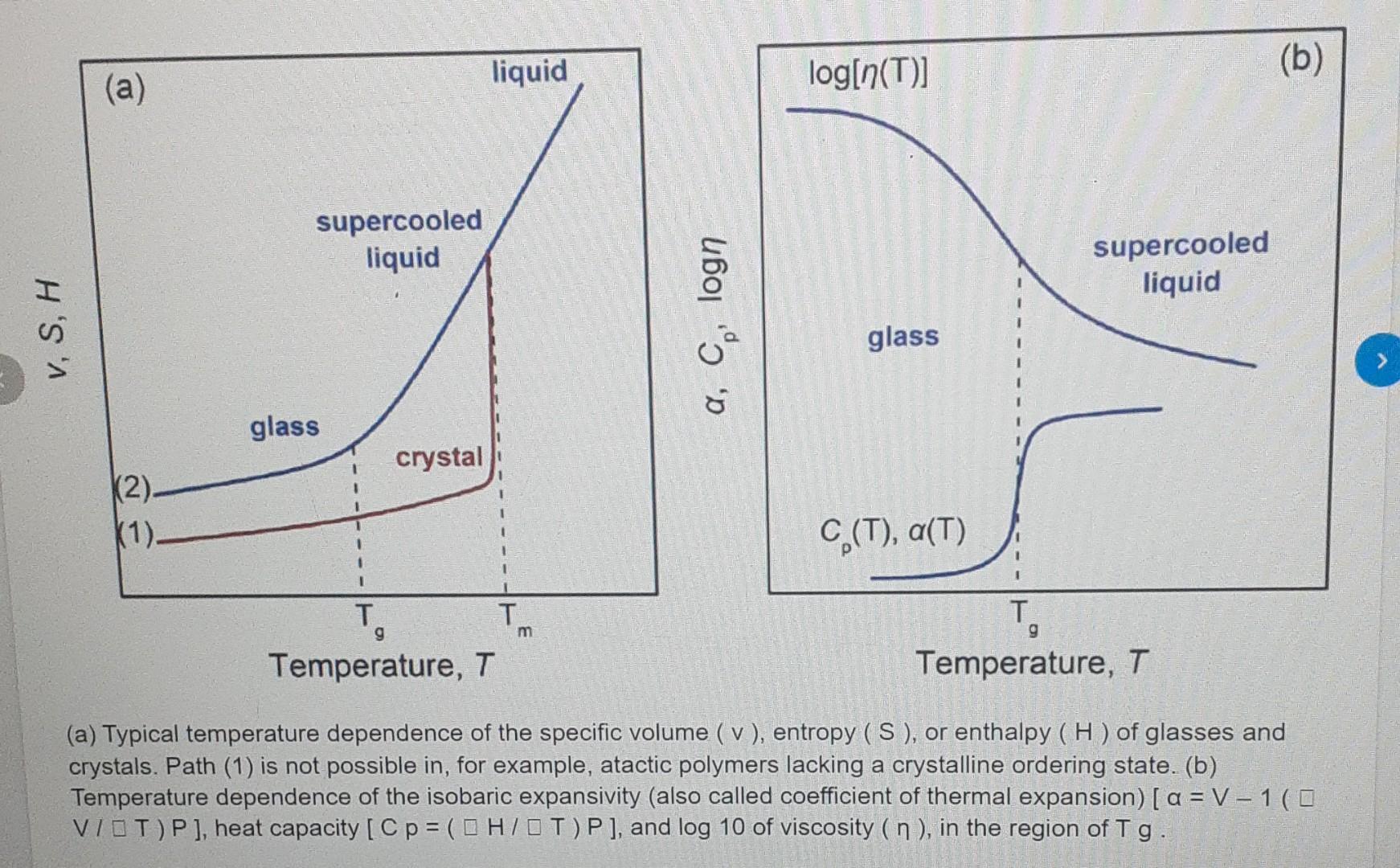
Polymer Engineering Find the transition temperature of the glass with the specified volume having the equation V() (L) = 1.137 + 1.4 X 10^-4 T (at low temperature) V(s) (H) = 1.145 + 8.0 X 10-^ -4 T(at high temperature) liquid (a) log[n(T)] (b) supercooled liquid supercooled liquid V, S, H a, Cologn glass glass crystal K2). (1) C (T), a(T) 1 T g m 9 . T. Temperature, T Temperature, T (a) Typical temperature dependence of the specific volume ( v), entropy (S), or enthalpy (H) of glasses and crystals. Path (1) is not possible in, for example, atactic polymers lacking a crystalline ordering state. (b) Temperature dependence of the isobaric expansivity (also called coefficient of thermal expansion) [a = V-100 VIOT)P], heat capacity [p = ( OH7OT)P], and log 10 of viscosity ( n ), in the region of Tg
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


