Answered step by step
Verified Expert Solution
Question
1 Approved Answer
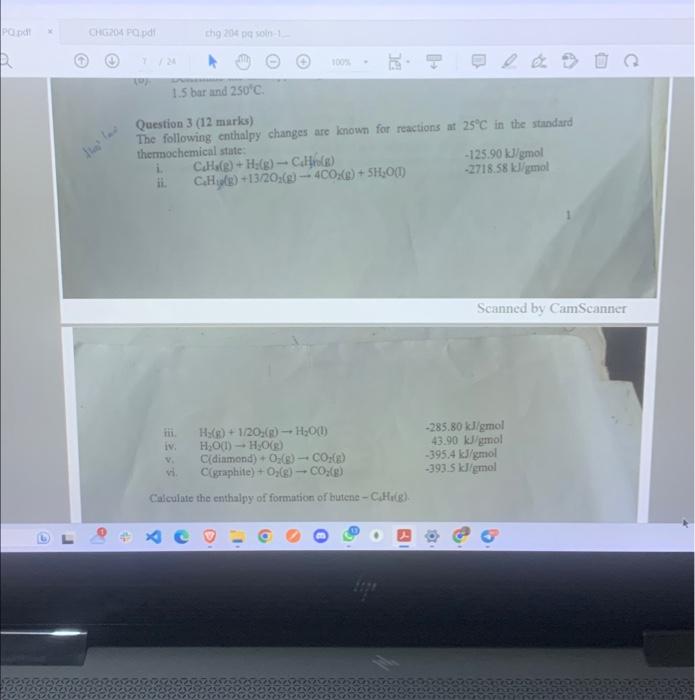
PQ.pdf X CHG204 PQ.pdf Hess' (av law 7 / 24 chg 204 pq soln-1.... 1.5 bar and 250C. i. ii. Question 3 (12 marks) The
PQ.pdf X CHG204 PQ.pdf Hess' (av law 7 / 24 chg 204 pq soln-1.... 1.5 bar and 250C. i. ii. Question 3 (12 marks) The following enthalpy changes are known for reactions at 25C in the standard thermochemical state: iii. iv. 100% H(g) + 1/2O2(g) HO(1) HO(1) HO(g) - CO(g) C(diamond) + O(g) - C(graphite) + O2(g) CO(g) Calculate the enthalpy of formation of butene - C4H8(g). V. vi. C4H8(g) + H(g) C4H(g) C4H10(g) +13/2O2(g) 4CO(g) + 5HO(1) B O -125.90 kJ/gmol -2718.58 kJ/gmol -285.80 kJ/gmol 43.90 kJ/gmol -395.4 kJ/gmol -393.5 kJ/gmol Scanned by CamScanner 465 1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


