Question
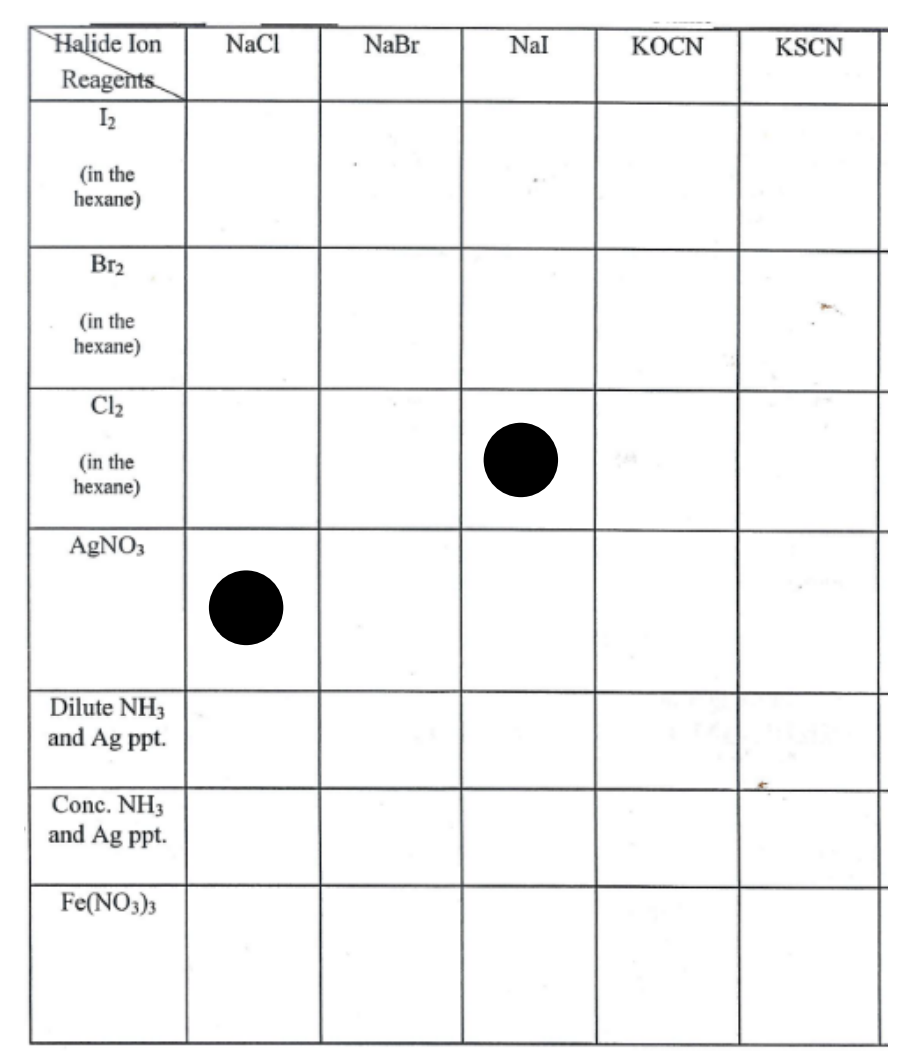
Pre Lab-06 Please read the lab procedure (Lab -06) before starting this assignment. You are given the following table. Write chemical equations to represent the
Pre Lab-06
Please read the lab procedure (Lab -06) before starting this assignment. You are given the following table. Write chemical equations to represent the possible product formation between the given halide ion solutions and the reagents. I will discuss your answers before the lab class. Examples Cl2 (in hexane) + 2NaI (aq) 2NaCl (aq) + I2 (in hexane) AgNO3 (aq) + NaCl (aq) AgCl(s) + NaNO3(aq)
LAB 6 - HALIDE ANALYSIS Introduction: On the periodic table groups of elements with similar chemical properties are found in vertical columns. The halogen family is column VII A and includes fluorine, chlorine, bromine, iodine, and astatine. In this activity the trends within the family will be examined. Procedure: The Five Solutions are: NaCl, NaBr, Nal, KSCN, and KOCN. These five plus your unknown are used for each of the following steps; RECORD the results in a DATA TABLE. A. 1. Place 7 mL of iodine (l2) water and 7 mL of organic solvent (hexane) into your French bottle with a dropper top. Close and shake to extract the color of l2 into the organic (i.e., top) layer. 2. Transfer a dropper full of the organic (i.e., top) layer to each of six 13x100 mm test tubes. Add 1 mL of one of the five solutions (NaCl, NaBr, Nal, KSCN, KOCN) separately to each of the first five tubes and 1 mL of your unknown to the last tube. 3. Mix the two layers in each test tube thoroughly and record the change in the color of the organic (i.e., top) layer. DISCARD ALL MIXTURES (In the French bottle and all test tubes) IN THE BOTTLES MARKED HALOGEN/ORGANIC WASTE. 4. Rinse out your French bottle and all test tubes with distilled water for the next procedure. B. Repeat steps 1-4 using bromine (Br2) water in place of I2 water. C. Repeat steps 1-4 using 5 mL of Chlorox and 10 drops of conc. HCl in place of I2 water. MAKE SURE THE HEXANE IS IN THE BOTTLE BEFORE YOU ADD THE HCI! This procedure will make chlorine (Cl2) water. D. 1. Place 5 drops of one of the five solutions (NaCl, NaBr, Nal, KOCN, KSCN) separately into each of the five 13 x 100 mm test tubes and 5 drops of your unknown to the last tube. 2. Add 5 drops of AgNO3 solution to each tube and mix thoroughly. Afterwards centrifuge the mixture. Record the color and appearance of the precipitate. 3. Now add 1 mL of distilled water along with 5 drops of concentrated ammonia to each of the six tubes. Mix thoroughly. Record if some or all of the precipitate dissolves in this dilute ammonia solution. Centrifuge any tube in which precipitate remains. 4. If a precipitate is present in any test tube, pour off the supernate (i.e., the liquid above the precipitate) and add 5 drops of concentrated ammonia directly to the precipitate. Mix thoroughly. Record the solubility of these precipitates in the concentrated ammonia solution. PLACE SILVER WASTE IN THE MARKED CONTAINER. E. 1. Place 5 drops of one of the five solutions (NaCl, NaBr, Nal, KOCN, KSCN) separately into each of five 13 x 100 mm test tubes and 5 drops of your unknown to the last tube. 2. Add 5 drops of Fe(NO3)3 solution to each tube and mix thoroughly. Record the color and appearance of the resulting solution. When finished, these iron containing solutions can be discarded down the sink.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


