Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The most abundant radon isotope is radon-222, which is an alpha emitter with a half-life of 3.8 days. The air in a house basement
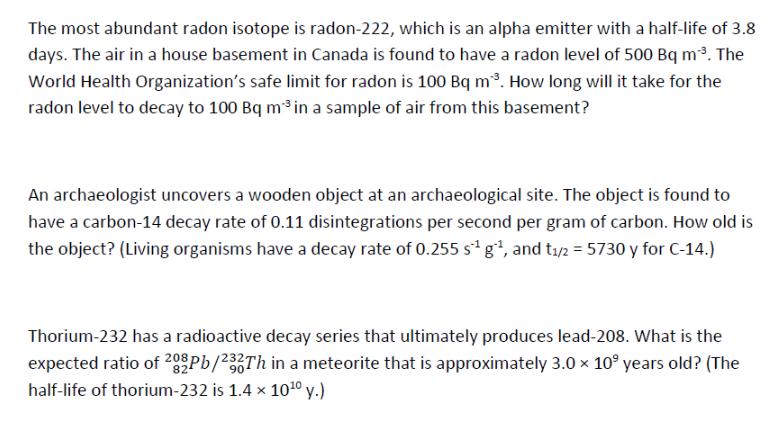
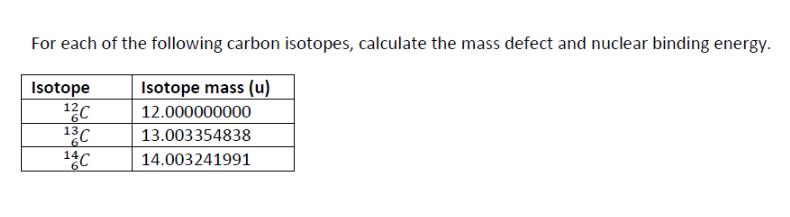
The most abundant radon isotope is radon-222, which is an alpha emitter with a half-life of 3.8 days. The air in a house basement in Canada is found to have a radon level of 500 Bq m. The World Health Organization's safe limit for radon is 100 Bq m. How long will it take for the radon level to decay to 100 Bq m in a sample of air from this basement? An archaeologist uncovers a wooden object at an archaeological site. The object is found to have a carbon-14 decay rate of 0.11 disintegrations per second per gram of carbon. How old is the object? (Living organisms have a decay rate of 0.255 s g, and t/2 = 5730 y for C-14.) Thorium-232 has a radioactive decay series that ultimately produces lead-208. What is the expected ratio of 202Pb/232Th in a meteorite that is approximately 3.0 109 years old? (The half-life of thorium-232 is 1.4 x 1010 y.) For each of the following carbon isotopes, calculate the mass defect and nuclear binding energy. Isotope 12C Isotope mass (u) 12.000000000 13C 13.003354838 6 14C 14.003241991
Step by Step Solution
★★★★★
3.53 Rating (143 Votes )
There are 3 Steps involved in it
Step: 1
1 Radon222 decay in the basement Radon222 has a halflife of 38 days The initial radon level is 500 B...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


