Question
a) Using the Lewis/Randall rule; f, xf, prove that , = 0, b) i) Determine the fugacity coefficient of methyl acetate at 523 K
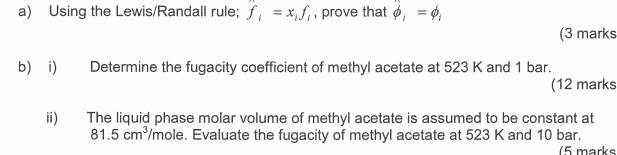
a) Using the Lewis/Randall rule; f, xf, prove that , = 0, b) i) Determine the fugacity coefficient of methyl acetate at 523 K and 1 bar. (3 marks (12 marks The liquid phase molar volume of methyl acetate is assumed to be constant at 81.5 cm/mole. Evaluate the fugacity of methyl acetate at 523 K and 10 bar. (5 marks
Step by Step Solution
3.43 Rating (169 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Operations Management
Authors: William J Stevenson
12th edition
2900078024107, 78024102, 978-0078024108
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



