Question
The compound acetone has two resonance structures which both represent a possible correct Lewis structure of the compounds (only one is shown below). Answer

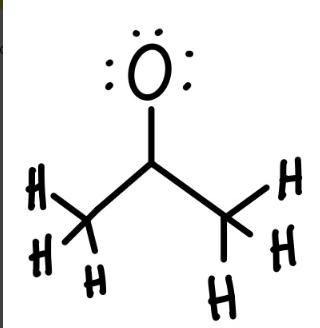
The compound acetone has two resonance structures which both represent a possible correct Lewis structure of the compounds (only one is shown below). Answer the following question regarding the structural depiction of Acetone. What are the formal charges (if any) of the oxygen and "carbonyl" (center in this case) carbon in this resonance structure? All of the hydrogens and lone pairs of electrons are drawn in. 0=+1;C=-1 OO=0C=0 0--1; C = +1 00--1;C=0 :: H H I. H = H
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Advanced Accounting
Authors: Joe Hoyle, Thomas Schaefer, Timothy Doupnik
10th edition
0-07-794127-6, 978-0-07-79412, 978-0077431808
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



