Answered step by step
Verified Expert Solution
Question
1 Approved Answer
previous wrong answers are: 1.3x10^-7, 142.5, and 1.3x10^-4 MISSED THIS? Read Section 15.5; Watch Determine the activation barrier for the reaction. A reaction has a
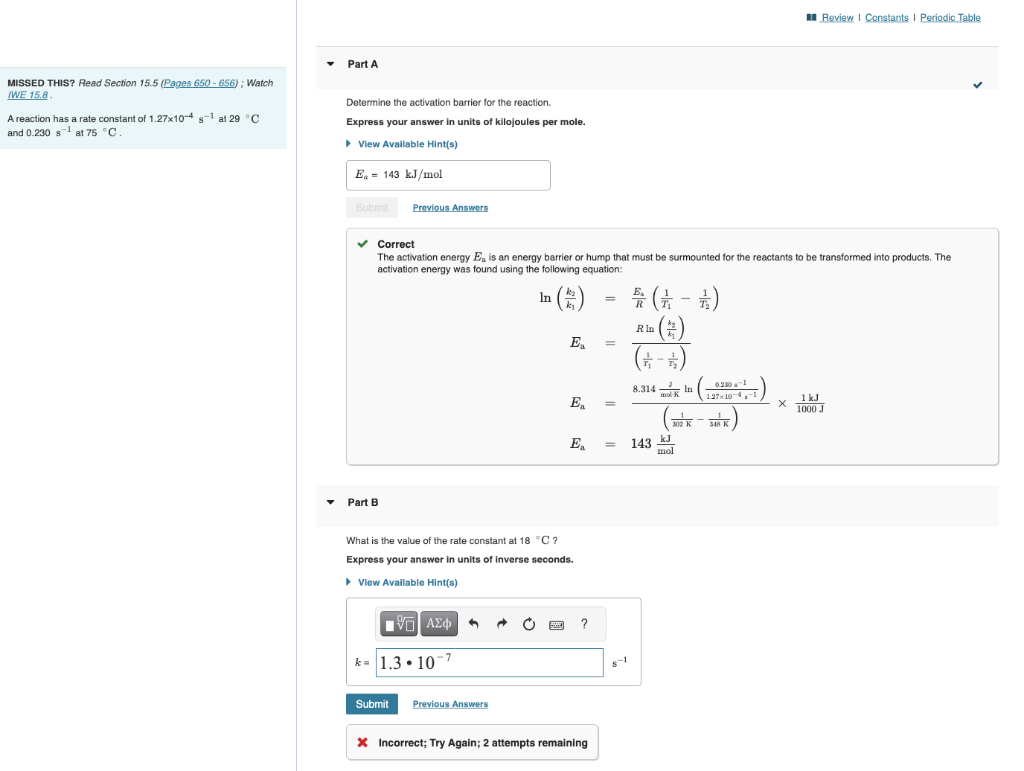
previous wrong answers are: 1.3x10^-7, 142.5, and 1.3x10^-4
MISSED THIS? Read Section 15.5; Watch Determine the activation barrier for the reaction. A reaction has a rate constant of 1.27104s1 at 29C Express your answer in units of kilojoules per mole. and 0.230s1 at 75C. Correct The activation energy Ea is an energy barrier or hump that must be surmounted for the reactants to be transformed into products. The activation energy was found using the following equation: ln(k1k2)EaEaEa=REa(T11T21)=(T11T21)Rln(k1k2)=(302K1368K1)8.314modKJln(1.27104s10.25a1)1000J1kJ=143molkJ Part B What is the value of the rate constant at 18C ? Express your answer in units of inverse seconds. x Incorrect; Try Again; 2 attempts remainingStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


