Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Prob. 13 (25 pts.) For each of the following fictitious compounds, use Pauling's equation to predict the percentage ionic character. NOTE: THE ANSWER KEY

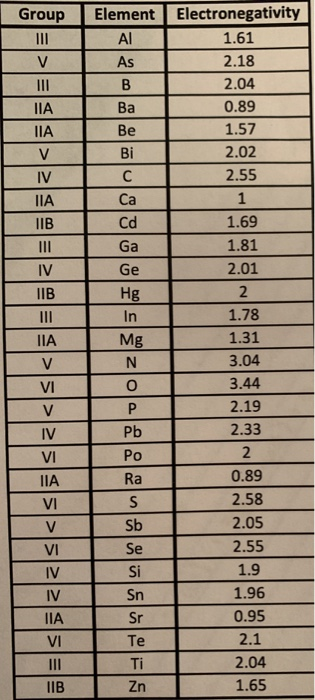
Prob. 13 (25 pts.) For each of the following fictitious compounds, use Pauling's equation to predict the percentage ionic character. NOTE: THE ANSWER KEY IS BASED ON THE TABLE OF ELECTRONEGATIVIES included in the ZIP folder (AA Electronegativities of Select Elements.PDF). Use any other source at your own risk! (a) Znin3 (c) Beln3 (e) Hg3Pb (b) CaC2 (d) Mg3Pb Group Element Electronegativity III 1.61 V As 2.18 III B 2.04 Ba 0.89 Be 1.57 V Bi 2.02 IV C 2.55 Ca 1 IIB Cd 1.69 III Ga 1.81 IV Ge 2.01 IIB Hg 2 III In 1.78 Mg 1.31 V N 3.04 VI 0 3.44 V P 2.19 IV Pb 2.33 VI Po 2 Ra 0.89 VI S 2.58 V Sb 2.05 VI Se 2.55 IV Si 1.9 IV Sn 1.96 Sr 0.95 VI Te 2.1 Ti 2.04 IIB Zn 1.65
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


