Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 1 ( 1 2 questions, 6 points each, Total 7 2 points ) The table below gives the hydrocarbons which have been identified in
Problem questions, points each, Total points
The table below gives the hydrocarbons which have been identified in the gasoline fraction of a crude oil. Identify the paraffins, naphthenes, and aromatics. Explain your answer by following the example given in the hint points each for explanation
Hydrocarbon
Methylcyclohexane
Methylbutane Isopentane
Cyclopentane
Methylbenzene Toluene
Methylcyclopentane
Dimethylpropane Neopentane
PETR SPRING University of Houston
Dimethylcyclohexane
Methylpentane Isohexane
Dimethylheptane
Dimethylbenzene paraXylene Xylene
Dimethylbenzene metaXylene Xylene
Dimethylbenzene orthoXylene oXylene
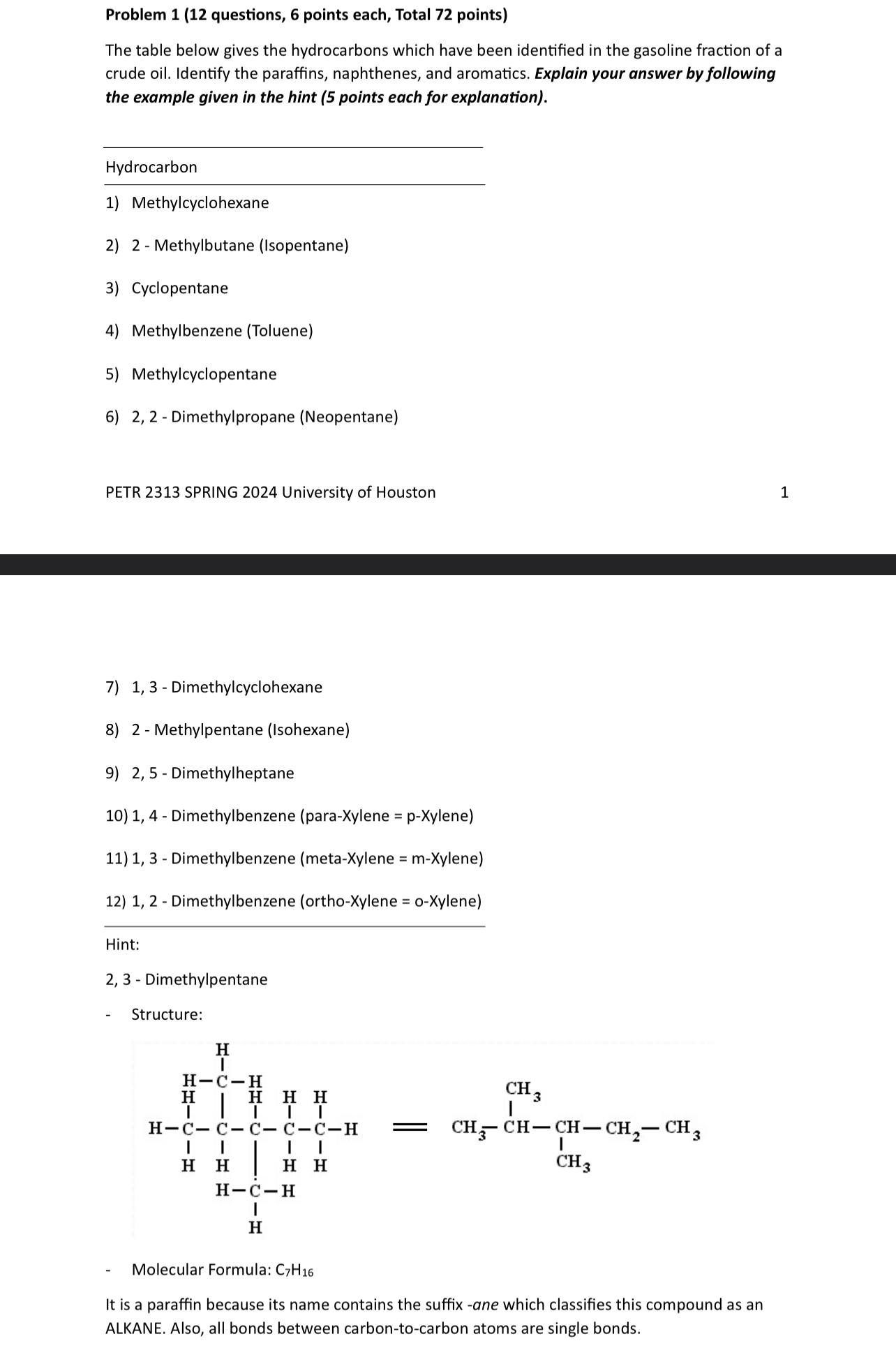
Hint:
Dimethylpentane
Structure:
Molecular Formula:
It is a paraffin because its name contains the suffix ane which classifies this compound as an ALKANE. Also, all bonds between carbontocarbon atoms are single bonds.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


