Answered step by step
Verified Expert Solution
Question
1 Approved Answer
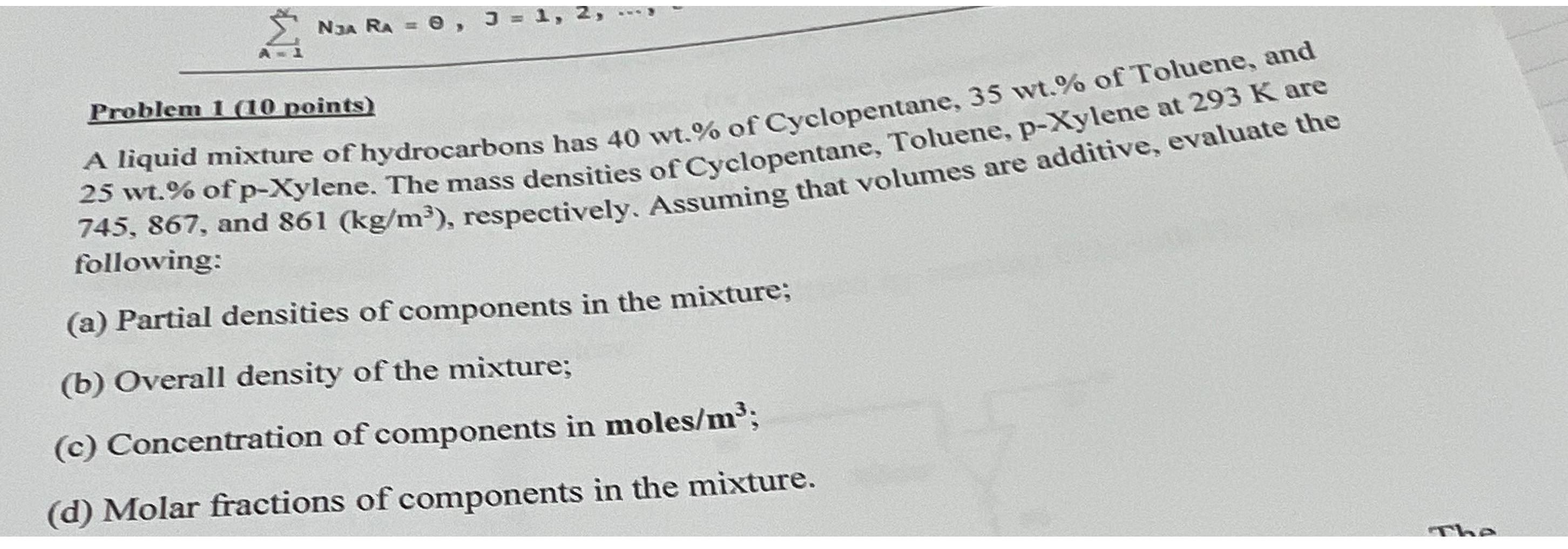
Problem 1 (10 points) A liquid mixture of hydrocarbons has 40wt.% of Cyclopentane, 35wt.% of Toluene, and 25wt% of p-Xylene. The mass
\ \ \ Problem 1 (10 points)\ A liquid mixture of hydrocarbons has
40wt.%of Cyclopentane,
35wt.%of Toluene, and
25wt%of p-Xylene. The mass densities of Cyclopentane, Toluene, p-Xylene at
293Kare 745,867 , and
861((kg)/(m^(3))), respectively. Assuming that volumes are additive, evaluate the following:\ (a) Partial densities of components in the mixture;\ (b) Overall density of the mixture;\ (c) Concentration of components in moles/
m^(3);\ (d) Molar fractions of components in the mixture.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


