Question
Problem 1: [30 points] A piston-cylinder system containing 0.5 Ibm of air undergoes two reversible processes as shown in the T-s diagram below. The
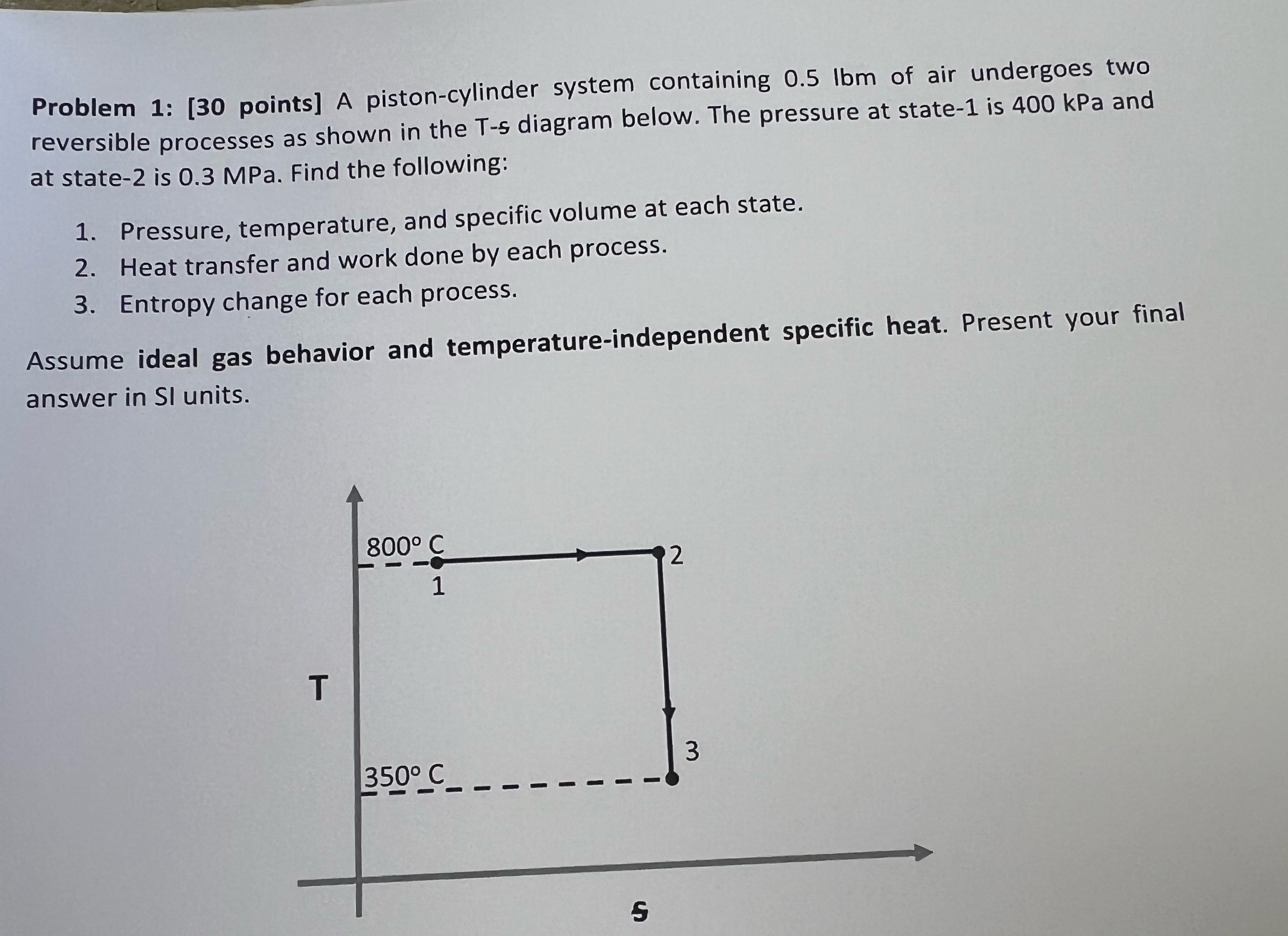
Problem 1: [30 points] A piston-cylinder system containing 0.5 Ibm of air undergoes two reversible processes as shown in the T-s diagram below. The pressure at state-1 is 400 kPa and at state-2 is 0.3 MPa. Find the following: 1. Pressure, temperature, and specific volume at each state. 2. Heat transfer and work done by each process. 3. Entropy change for each process. Assume ideal gas behavior and temperature-independent specific heat. Present your final answer in SI units. T 800 C 1 2 3 350 C S
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



