Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 1 a . Derive the equation d U = [ C p - P ( d e l V d e l T )
Problem
a Derive the equation
b Determine for an ideal gas using the expression in part a Does depend on
temperature or pressure or both?
c Let and Using the van der Waals equation of state
derive expressions for and For a van der Waals gas, does
depend on temperature or pressure or both?
d Derive an expression for in terms of and
e For a van der Waals gas, derive the expression for enthalpy change associated with an
isothermal expansion from to How does this compare with the enthalpy change
of an ideal gas undergoing isothermal expansion from to
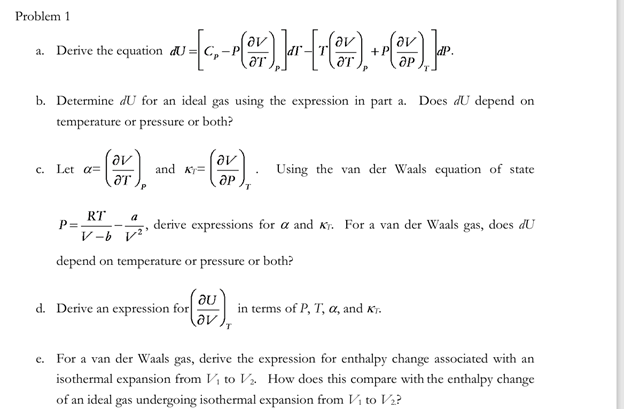
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


