Answered step by step
Verified Expert Solution
Question
1 Approved Answer
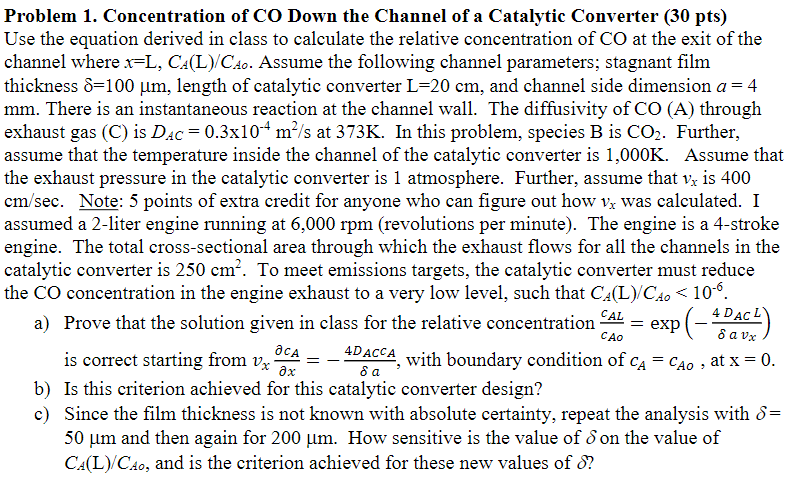
Problem 1 . Concentration of CO Down the Channel of a Catalytic Converter ( 3 0 p t s Use the equation derived in class
Problem Concentration of CO Down the Channel of a Catalytic Converter
Use the equation derived in class to calculate the relative concentration of at the exit of the
channel where Assume the following channel parameters; stagnant film
thickness length of catalytic converter and channel side dimension
There is an instantaneous reaction at the channel wall. The diffusivity of through
exhaust gas C is at In this problem, species is Further,
assume that the temperature inside the channel of the catalytic converter is Assume that
the exhaust pressure in the catalytic converter is atmosphere. Further, assume that is
Note: points of extra credit for anyone who can figure out how was calculated. I
assumed a liter engine running at revolutions per minute The engine is a stroke
engine. The total crosssectional area through which the exhaust flows for all the channels in the
catalytic converter is To meet emissions targets, the catalytic converter must reduce
the concentration in the engine exhaust to a very low level, such that
a Prove that the solution given in class for the relative concentration exp
is correct starting from with boundary condition of at
b Is this criterion achieved for this catalytic converter design?
c Since the film thickness is not known with absolute certainty, repeat the analysis with
and then again for How sensitive is the value of on the value of
and is the criterion achieved for these new values of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


