Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem #1: Consider a known mass of oxygen in an unconstrained (i.e., isobaric) piston cylinder. The mass is 0.78 kg. The initial conditions are
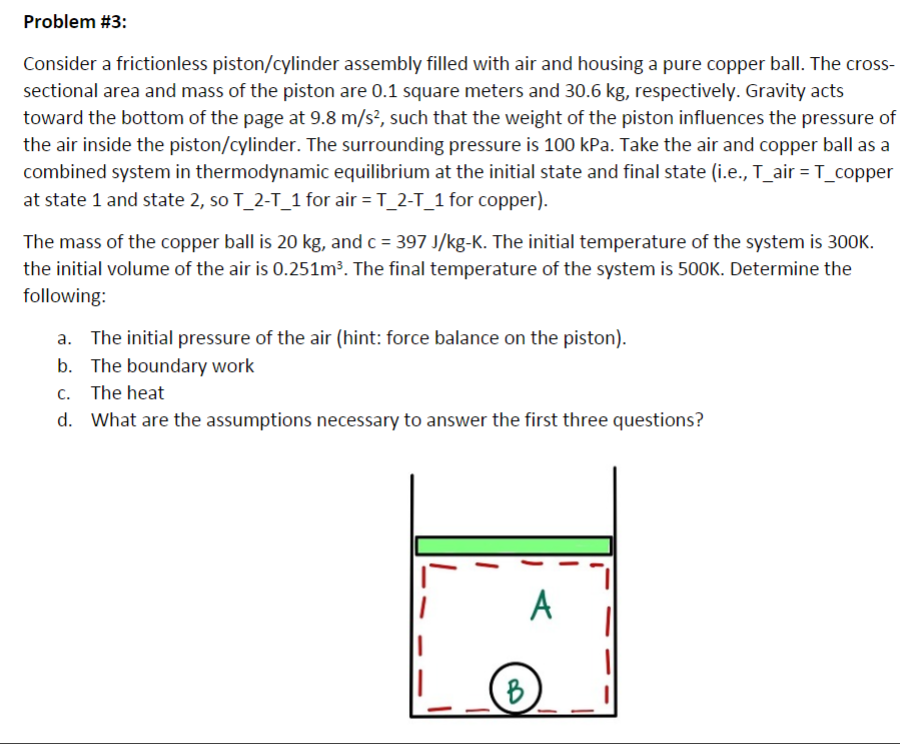
Problem #1: Consider a known mass of oxygen in an unconstrained (i.e., isobaric) piston cylinder. The mass is 0.78 kg. The initial conditions are 400K and 200 kPa. The final temperature is 500K. Determine the following: a. Boundary Work b. Heat c. Draw the process on a P-v diagram d. Draw the process on a T-v diagram Problem #2: Consider R-134a from 200kPa and 10 Celsius compressed in a polytropic manner (it is not and ideal gas, but a superheated vapor can still go through a polytropic process), in which n = 1.4, to a final pressure of 400kPa. a. Determine the initial specific volume b. Determine the final specific volume c. Determine the work per mass d. Determine the heat per mass e. Draw the process on a P-v diagram Problem #3: Consider a frictionless piston/cylinder assembly filled with air and housing a pure copper ball. The cross- sectional area and mass of the piston are 0.1 square meters and 30.6 kg, respectively. Gravity acts toward the bottom of the page at 9.8 m/s, such that the weight of the piston influences the pressure of the air inside the piston/cylinder. The surrounding pressure is 100 kPa. Take the air and copper ball as a combined system in thermodynamic equilibrium at the initial state and final state (i.e., T_air = T_copper at state 1 and state 2, so T_2-T_1 for air = T_2-T_1 for copper). The mass of the copper ball is 20 kg, and c = 397 J/kg-K. The initial temperature of the system is 300K. the initial volume of the air is 0.251m. The final temperature of the system is 500K. Determine the following: a. The initial pressure of the air (hint: force balance on the piston). b. The boundary work c. The heat d. What are the assumptions necessary to answer the first three questions? B A
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


