Problem 1: For the HDA process, generate a detailed P&ID for the fired heater (H-101)
based on the lecture notes of HAZOP study and analysis.
Problem 2: For the attached acrylic acid process, answer the following questions:
(1) Determine the number of stages and the compression ratio required to compress the air feed
stream. Calculate the air temperature after each stage of compressing w/o intercooling?
Given: Tout = Tin [1+{(Pout/Pin)
(-1)/
-1}/]; assume = 1.4, = 0.7, T in K.
(2) Write the energy balance equation for the reactor and define each term in the balance equation.
What information do you need to solve that equation? No numerical calculations are required.
(3) Draw a P&ID for the isothermal reactor if its temperature is to be controlled by manipulating
the cooling water flow rate used to cool the molten salt in its cycle.
(4) Justify why steam is fed into the reactor. Find the concentration of C3H6 in air in presence of
steam and in absence of steam then find suitable justification. Comment on results knowing
that the explosive limits for C3H6 in air are 2.1% to 12.1% at ambient conditions.
(5) Why is the reactor operated at an elevated temperature of 310C? Also, why the reactor is not
operated at temperatures above 310oC? Discuss and justify each case.
(6) What would be the effect of using higher pressure in the reactor (say, 10 bar instead of 3.5
bar)? Explain your answer.
(7) Is there any conditions of special concern in the reactor effluent (product) separation system?
Mention then, justify and sate the penalties of operating under such conditions.
(8) It has been proposed that a portion of the off-gas stream be recycled back to the reactor while
the amount of steam feed is reduced. Can you foresee any problems or areas of special concern
if this were implemented?
(9) Check the quality of the feed stream (14) to the distillation tower (T-1005), i.e., find the bubble
point of that stream and comment on its quality.
(10) Find how much heat is removed from the reactor by making energy balance on the molten
salt cooler (E-1001).
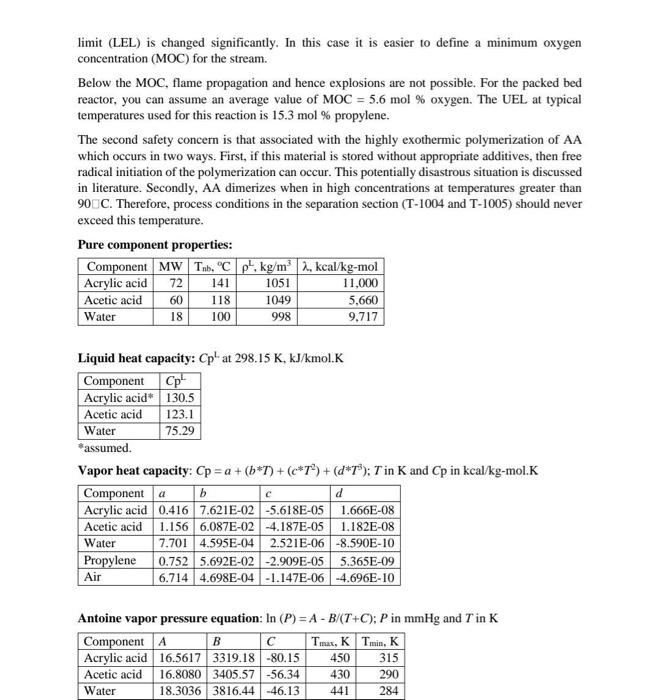
Acrylic Acid production by catalytic partial oxidation of propylene
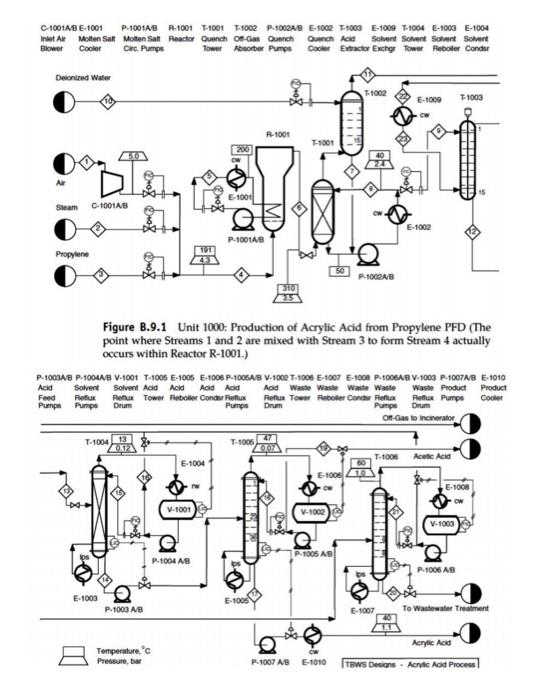
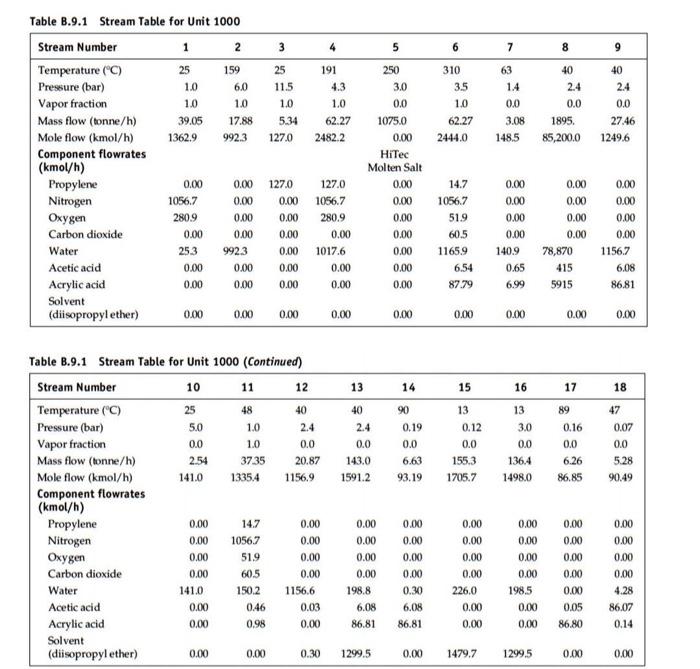
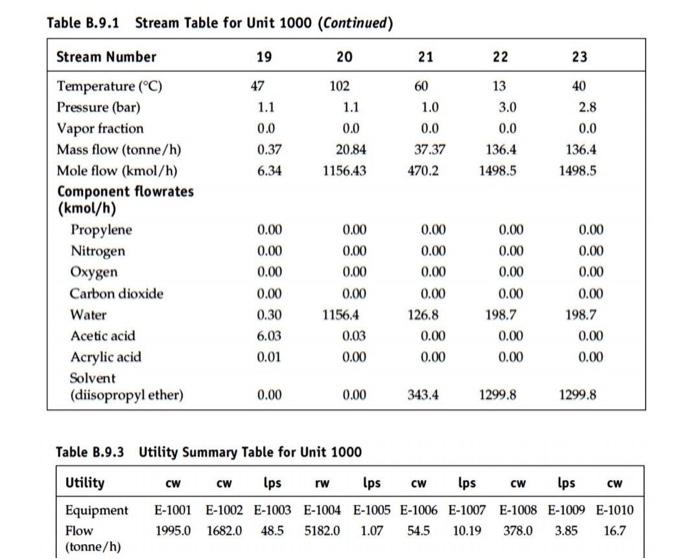
6:30 Telegram Answer 1 of 1 Done Chromosome condensation -c)prophase In prophase chromosomal material condense to form compact mitotic chromosome. Alignment of chromosome at poles- D) Anaphase In anaphase chromosome split and chromatid moves to opposite poles. Assembly of chromosome in metaphase plate-E)Metaphase Appearance of cell plate-F)plant cell In plant cell during cytokinesis wall formation starts in the centre of the cell and grows outward to meet the existing lateral wall. Formation of new cell wall begins with formation of cell plate. Claevage furrow-A)Animal cell In animal cell cytokinesis is achieved by the appearance of furrow in plasma membrane. Formation of nuclear membrane- B)Telophase In telophase nuclear membrane assebles around the chromosome cluster. Reaction Section: Propylene is supplied as a saturated vapor from storage at 11.5 bars, Stream 3. After throttling to 4.3 bars, Stream 3 is mixed with low-pressure steam (6 bars, 159"). Stream 2 and air. Stream 1. which is compressed to 5 huis in compressor C-1001 prior to mixing. The resulting mixture, Stream 4. is fed to fluidized bed catalytic reactor R-1001 The fluidized bed operates close to isothermally at 310C with a single pass conversion of propylene of about 88%. The beat of reaction is removed by a circulating stream of molten alt which is cooled externally in exchanger E-1001 by cooling water. Three primary reactions take place in the reactor, giving rise to two organic acid products - acrylic and acetic acids Acrylic acid is produced by the catalytic oxidation of propylene with air 16 CH+15 0:+CHO (acrylic acid) + H2O (R) Along with the main reaction shown above, several additional oxidation reactions occur to yield a variety of by products, including acetic acid and Co CH+2.50 +C.H.O. Cacetic acid) +CO HO CH+4.50; +300+ 3H-0 (R) The rate is given by: The partial pressure p is in kPa. R = 1.987 kcal/kmol.K. The activation energy (E) and ki, are given below R. (kmol/m' reactor.hr) AkPa) E. kcal/kmol 1 1.59 x 10 15,000 2 8.83 x 10 20.000 3 1.81 x 10 25.000 Products Separation Section: The stream leaving the acrylic acid reactor. Suream 6, contains a variety of products by-products and unused reactants. In order to separate the non-condensable fraction of this stream, the reactor effluent is sent to two absorber Stream 6 is rapidly quenched by a circulating stream of water Stream in the Quench Tower. T-1001. Most of the organic acids dissolve in the water. The non- condensable gases plus small amounts of acrylic acid and acetic acid leave the top of T-101 and are led to the Quench Cooler, T-1002, where they are contacted with a countercurrent stream of desonized water. Stream 10. Most of the remaining acid is absorbed into the water stream, Stream 7, which leaves the bottom of T-1002 and is mixed with the quench water. The gases leaving T-1012 contain the non condensable gases, including the un-reacted propylene, and these are sent off site to an incinerator for disposal. The combined stream leaving the bottom of both absorbers Stream 9. is sent to a rotating disk, liquid-liquid extractor tower. T-1003. Here the organic acids are extracted from the Water using a solvent (disopropyl ether) Safety Considerations It is recommended that the steam-to-propylene ratio at the reactor inlet never be set less than 41 on a mole basis. This steam is used to inhibit the formation of coke on the catalyst at temperatures below 330C As with any reaction involving the partial oxidation of a fuel-like feed material (propylene) considerable attention must be paid to the composition of hydrocarbons and oxygen in the feed stream. This stream is a potential explosion hazard. Operation outside of the explosion limits (LEL and UEL) is strongly recommended for a packed bed reactor. The explosion limits for propylene in air at 25C are LEL = 2.1 mol propylene and UEL = 12.1 mol propylene. At elevated temperatures and using a 4:1 steam-o-propylene ratio (see above) in the feed, the lower explosion limit (LEL) is changed significantly. In this case it is easier to define a minimum oxygen concentration (MOC) for the stream. Below the MOC, flame propagation and hence explosions are not possible. For the packed bed reactor, you can assume an average value of MOC = 5.6 mol % oxygen. The UEL at typical temperatures used for this reaction is 15.3 mol % propylene. The second safety concern is that associated with the highly exothermic polymerization of AA which occurs in two ways. First, if this material is stored without appropriate additives, then free radical initiation of the polymerization can occur. This potentially disastrous situation is discussed in literature. Secondly, AA dimerizes when in high concentrations at temperatures greater than 90CC. Therefore, process conditions in the separation section (T-1004 and T-1005) should never exceed this temperature. Pure component properties: Component MW Tb, C p', kg/m 2. kcal/kg-mol Acrylic acid Acetic acid 1049 5,660 Water 9.717 1051 11,000 72 60 18 141 118 100 998 Liquid heat capacity: Cpat 298.15 K, kJ/kmol.K Component Cpu Acrylic acid* 130.5 Acetic acid 123.1 Water 75.29 *assumed. Vapor heat capacity: Cp =a +(b*T) + (c*T>) + (d*13); Tin K and Cp in kcal/kg-mol.K Component a b Acrylic acid 0.416 7.621E-02 -5.618E-05 1.666E-08 Acetic acid 1.156 6.087E-02 -4.187E-05 1.182E-08 Water 7.701 4.595E-04 2.521E-06 -8.590E-10 Propylene 0.752 5.692E-02 -2.909E-05 5.365E-09 Air 6.714 4.698E-04 -1.147E-06 -4.696E-10 C d Antoine vapor pressure equation: In (P) = A - B/(T+C); P in mmHg and T in K Component A B Tmax, KTmin, K Acrylic acid 16.5617 3319.18 -80.15 450 315 Acetic acid 16.8080 3405.57 -56.34 430 290 Water 18.3036 3816.44-46.13 441 284 C-1001AB E-1001 P-1001AB A-1001 T-1001 1002 P.1002AB E-100-1000 E-1009 F-1004 E-1000 E-1004 Inlet Air Morten San Molten Sal Reactor Quench on Gas Duerch Quench Acid Solvent Solvent Solvent Solvent Blower Cooler Circ. Pumpe Tower Absorber Pumps Cooler Extractor Exchg Tower Reboiler Condor Deionized Water F-1002 E-1000 T-1003 TH R-1001 T-1001 200 OW TI ni REDEEMIT E-1001 Steam C-100118 CW E-1002 P-1001AB Propylene P-1002B Figure B.9.1 Unit 1000 Production of Acrylic Acid from Propylene PFD (The point where Streams 1 and 2 are mixed with Stream 3 to form Stream 4 actually occurs within Reactor R-1001.) P-1003A/B P-1004AB V-1001 T-1005 E-1005 E-1006 P-1005A/B V-1002 T-1006 E-1007 E-1009 P-1006AB V-1003 P-1007AB E-1010 Acid Solvent Solvent Acid Acid Acid Add Add Waste Waste Waste Waste Waste Product Product Feed Reflux Reflux Tower Reboller Condo Reflux Reflux Tower Rebole Condu Reflux Reflux Pumps Cooler Pump Pumps Drum Pumps Drum Pumpe Drum On-Gas to Incinerator T-10041 T-1005 DOA T-1006 Acetic Acid E-1004 E-1006 E-1000 V-1001 V-1002 sal V-1005 P1005 AB P-1004 AB P.1006 AB E-1003 E-1004 P-1003 AB E-1007 To Wastewater Treatment Acrylic Acid CH Temperature.c Pressure, bar P-1007AB E-1010 TBWS Designs - Acrylic Aod Process 5 6 7 8 9 310 3.5 1.0 62.27 2444.0 63 1.4 0.0 3.08 148.5 40 2.4 0.0 40 2.4 0.0 27.46 1249.6 1895 85,200.0 Table B.9.1 Stream Table for Unit 1000 Stream Number 12 3 4 Temperature (C) 25 159 25 191 Pressure (bar) 1.0 6,0 11.5 4.3 Vapor fraction 1.0 10 1.0 1.0 Mass flow (tonne/h) 39.05 17.88 5.34 62.27 Mole flow (kmol/h) 1362.9 9923 127.0 24822 Component flowrates (kmol/h) Propylene 0.00 0.00 127.0 127.0 Nitrogen 1056.7 0.00 0.00 1056.7 Oxygen 2809 0.00 0.00 280.9 Carbon dioxide 0.00 0.00 0.00 0.00 25.3 992.3 0.00 1017.6 Acetic acid 0.00 0.00 0.00 0.00 Acrylic acid 0.00 0.00 0.00 0.00 Solvent (diisopropyl ether) 0.00 0.00 0.00 0.00 250 3.0 0.0 1075.0 0.00 HiTec Molten Salt 0.00 0.00 0.00 0.00 0.00 0.00 0.00 14.7 1056.7 519 60.5 1165.9 6.54 87.79 0.00 0.00 0.00 0.00 140.9 0.65 6.99 0.00 0.00 0.00 0.00 78,870 415 5915 0.00 0.00 0.00 0.00 1156.7 6.08 86.81 Water 0.00 0.00 0.00 0.00 0.00 13 14 15 16 17 18 40 2.4 90 0.19 13 3.0 89 0.16 0.0 0.0 13 0.12 0.0 155.3 1705.7 0.0 0.0 47 0.07 0.0 5.28 90.49 2.54 143.0 1591.2 6.63 93.19 136.4 1498.0 6.26 86,85 Table B.9.1 Stream Table for Unit 1000 (Continued) Stream Number 10 11 12 Temperature (C) 25 48 40 Pressure (bar) 5.0 1.0 2.4 Vapor fraction 0.0 1.0 0.0 Mass flow (tonne/h) 3735 20.87 Mole flow (kmol/h) 141.0 1335.4 1156.9 Component flowrates (kmol/h) Propylene 0.00 14.7 0.00 Nitrogen 0.00 10567 0.00 Oxygen 0.00 51.9 0.00 Carbon dioxide 0.00 60.5 0.00 Water 141.0 150.2 1156.6 Acetic acid 0.00 0.46 0.03 Acrylic acid 0,00 0.00 Solvent (diisopropyl ether) 0.00 0.00 0.30 0.00 0.00 0.00 0.00 0.00 198.8 6.08 86.81 0.00 0.00 0.00 0.30 6.08 86.81 0.00 0.00 0.00 0.00 226.0 0.00 0.00 0.00 0.00 0.00 0.00 198.5 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.05 86,80 0.00 0.00 0.00 0.00 4.28 86.07 0.14 0.98 1299.5 0.00 1479.7 1299.5 0.00 0.00 21 22 23 0.0 60 1.0 0.0 37.37 470.2 13 3.0 0.0 136.4 1498.5 40 2.8 0.0 136.4 1498.5 Table B.9.1 Stream Table for Unit 1000 (Continued) Stream Number 19 20 Temperature (C) 47 102 Pressure (bar) 1.1 1.1 Vapor fraction 0.0 Mass flow (tonne/h) 0.37 20.84 Mole flow (kmol/h) 6.34 1156.43 Component flowrates (kmol/h) Propylene 0.00 0.00 Nitrogen 0.00 0.00 Oxygen 0.00 0.00 Carbon dioxide 0.00 0.00 Water 0.30 1156.4 Acetic acid 6.03 0.03 Acrylic acid 0.01 0.00 Solvent (diisopropyl ether) 0.00 0.00 0.00 0.00 0.00 0.00 126.8 0.00 0.00 0.00 0.00 0.00 0.00 198.7 0.00 0.00 0.00 0.00 0.00 0.00 198.7 0.00 0.00 343.4 1299.8 1299.8 Ips CW Table B.9.3 Utility Summary Table for Unit 1000 Utility CW CW lps rw CW Ips lps CW Equipment E-1001 E-1002 E-1003 E-1004 E-1005 E-1006 E-1007 E-1008 E-1009 E-1010 Flow 1995.0 1682.0 48.5 5182.0 1.07 54.5 10.19 378.0 3.85 16.7 (tonne/h)