Answered step by step
Verified Expert Solution
Question
1 Approved Answer
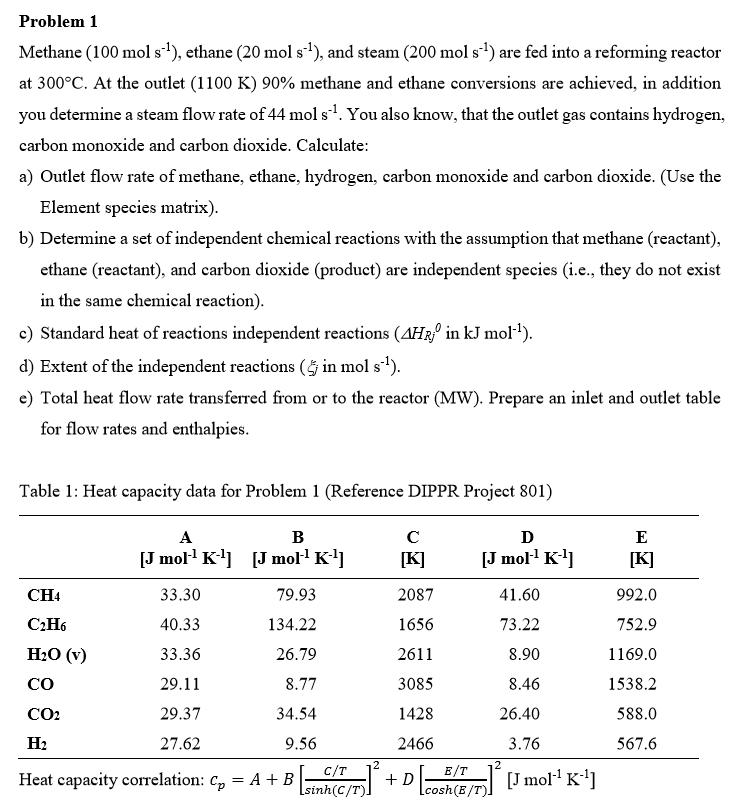
Problem 1 Methane ( 1 0 0 ( m o l ) s - 1 ) , ethane ( 2 0 ( m o l
Problem
Methane ethane and steam : are fed into a reforming reactor
at At the outlet methane and ethane conversions are achieved, in addition
you determine a steam flow rate of You also know, that the outlet gas contains hydrogen,
carbon monoxide and carbon dioxide. Calculate:
a Outlet flow rate of methane, ethane, hydrogen, carbon monoxide and carbon dioxide. Use the
Element species matrix
b Determine a set of independent chemical reactions with the assumption that methane reactant
ethane reactant and carbon dioxide product are independent species ie they do not exist
in the same chemical reaction
c Standard heat of reactions independent reactions in :
d Extent of the independent reactions in
e Total heat flow rate transferred from or to the reactor MW Prepare an inlet and outlet table
for flow rates and enthalpies.
Table : Heat capacity data for Problem Reference DIPPR Project
Heat capacity correlation:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


