Answered step by step
Verified Expert Solution
Question
1 Approved Answer
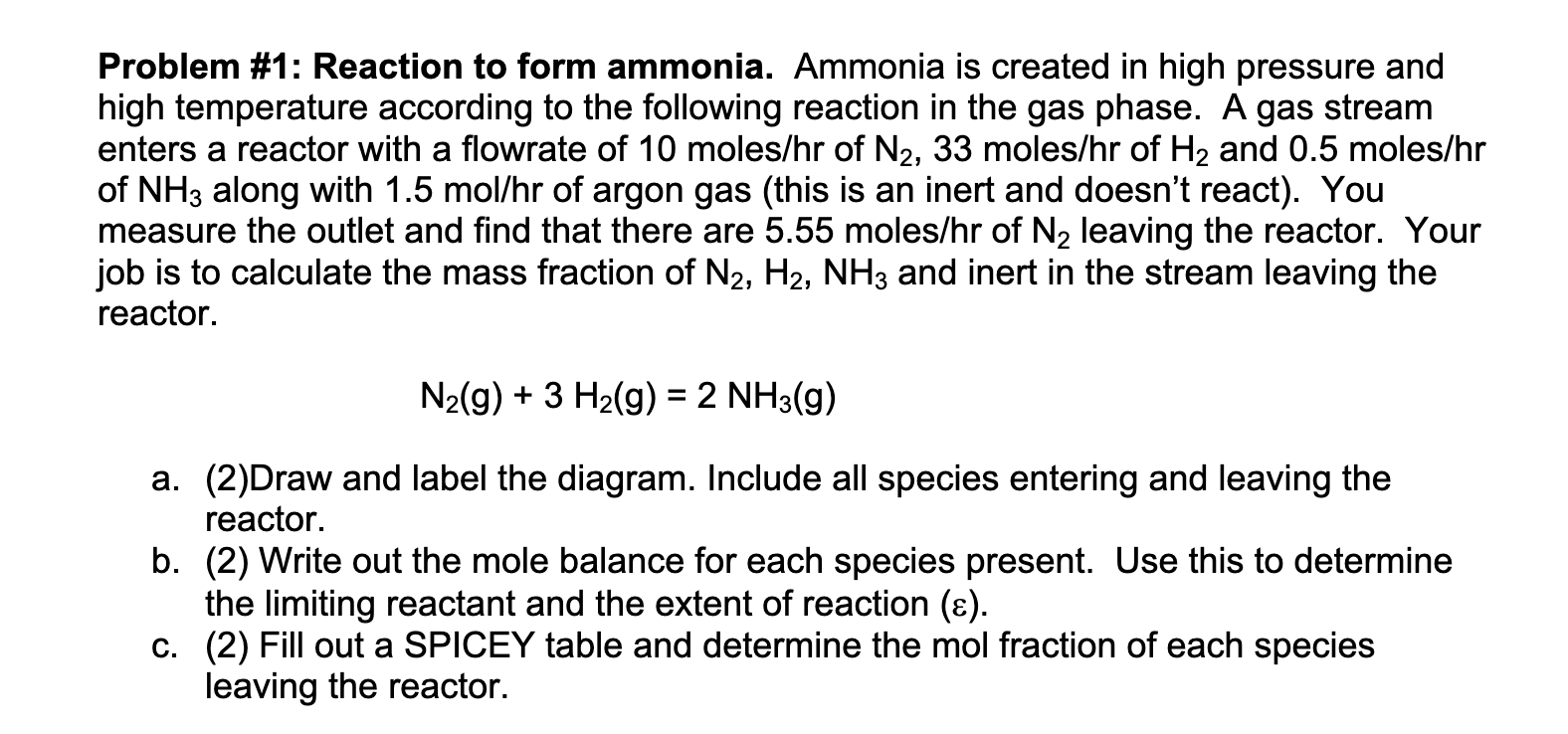
Problem # 1 : Reaction to form ammonia. Ammonia is created in high pressure and high temperature according to the following reaction in the gas
Problem #: Reaction to form ammonia. Ammonia is created in high pressure and
high temperature according to the following reaction in the gas phase. A gas stream
enters a reactor with a flowrate of moles of moles of and moles
of along with of argon gas this is an inert and doesn't react You
measure the outlet and find that there are moles of leaving the reactor. Your
job is to calculate the mass fraction of and inert in the stream leaving the
reactor.
aDraw and label the diagram. Include all species entering and leaving the
reactor.
b Write out the mole balance for each species present. Use this to determine
the limiting reactant and the extent of reaction
c Fill out a SPICEY table and determine the mol fraction of each species
leaving the reactor.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


