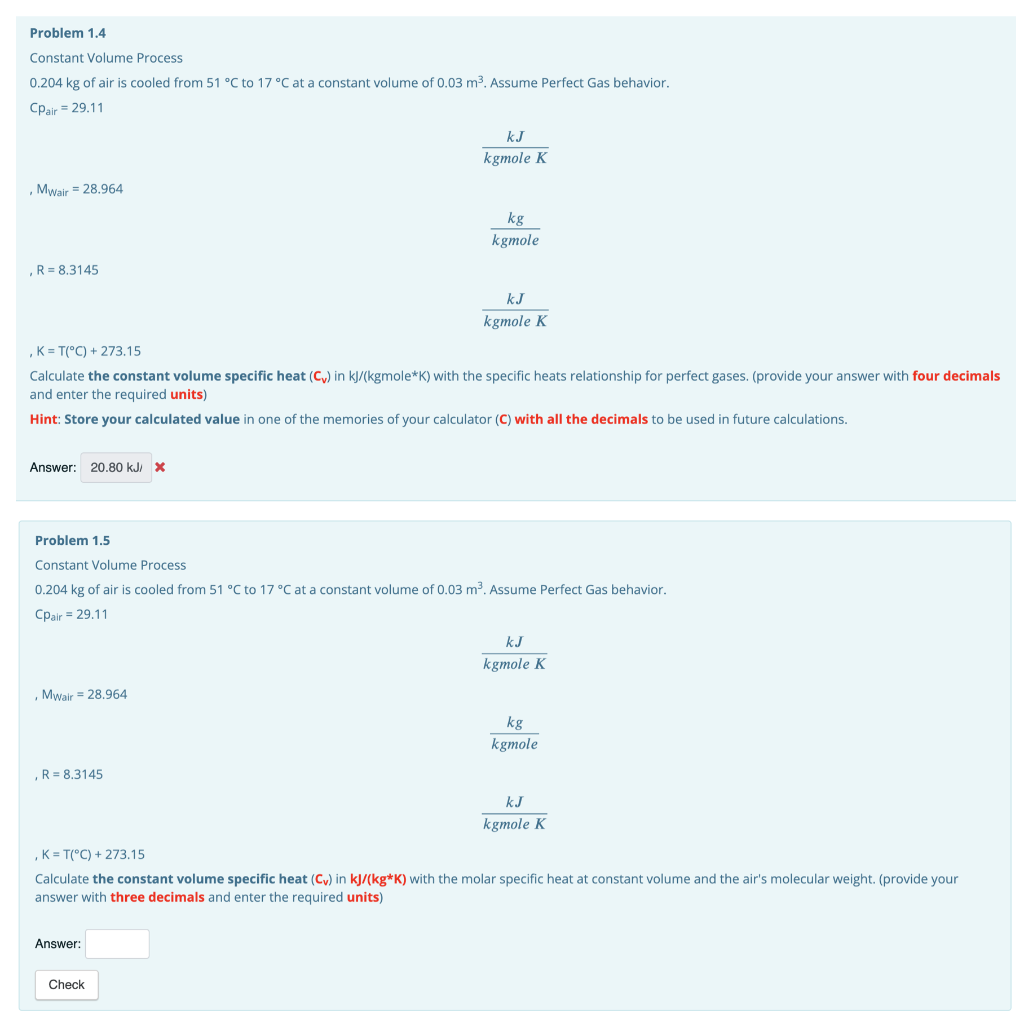
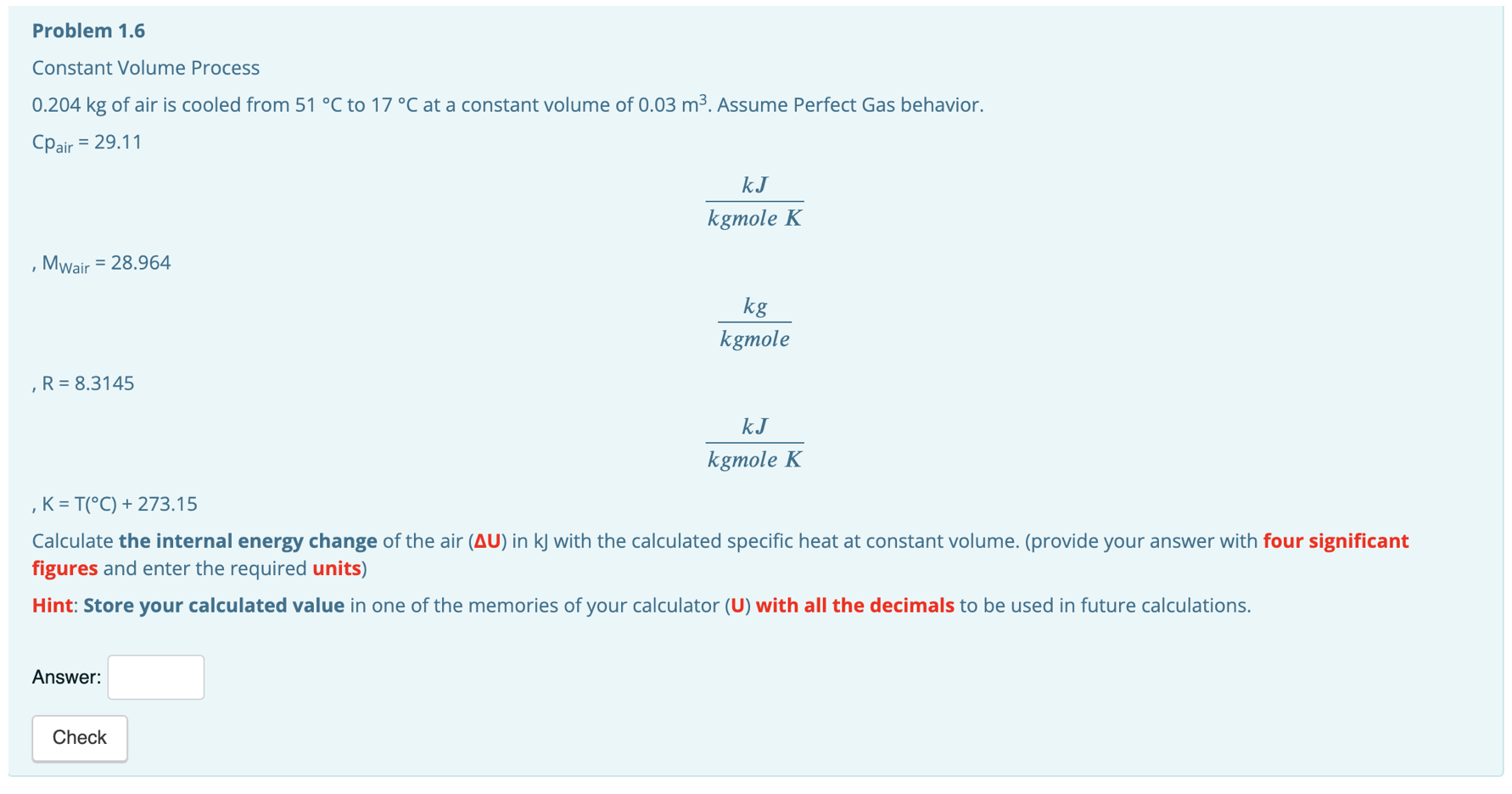
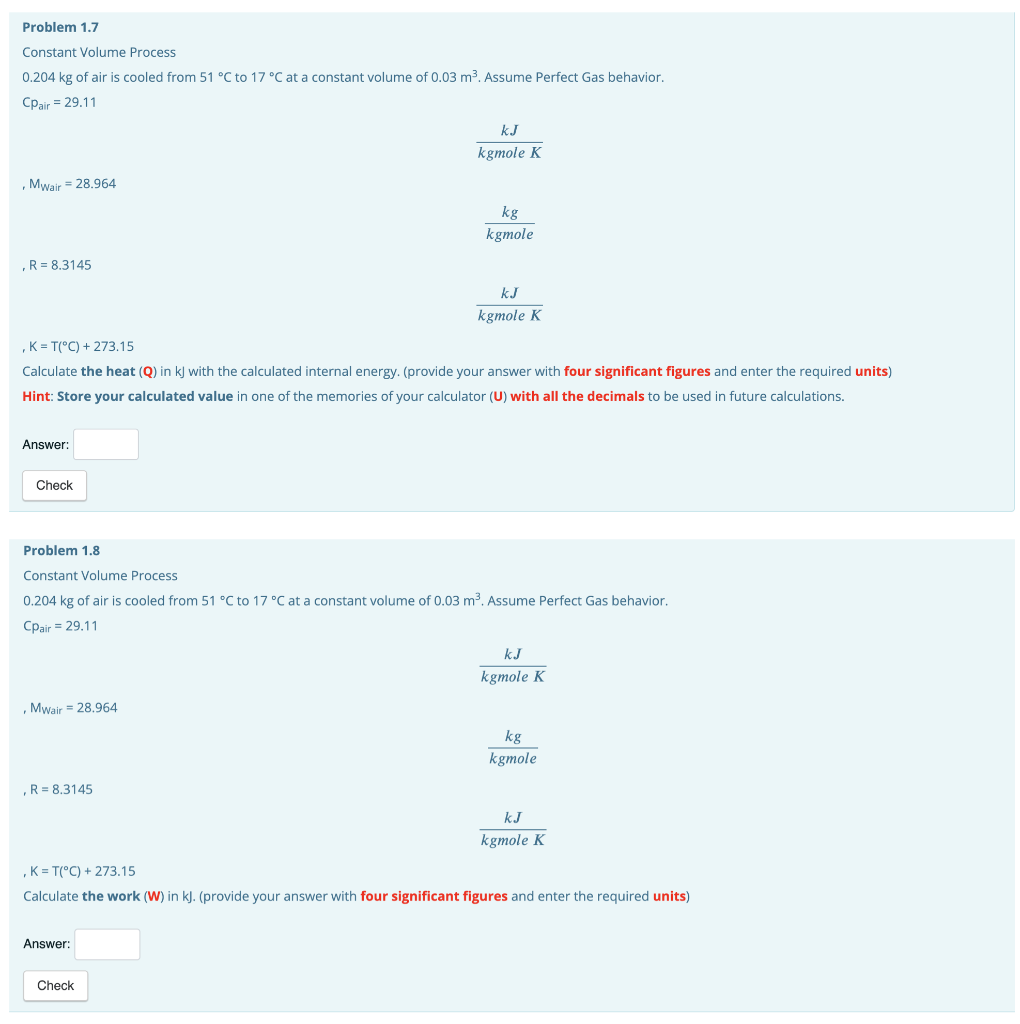
Problem 1.4 Constant Volume Process 0.204 kg of air is cooled from 51 C to 17C at a constant volume of 0.03 m2. Assume Perfect Gas behavior. Cpair = 29.11 k kgmole K Mwair = 28.964 kg kgmole R = 8.3145 kJ kgmole K K = T(C) + 273.15 Calculate the constant volume specific heat (Cy) in kJ/(kgmole*K) with the specific heats relationship for perfect gases. (provide your answer with four decimals and enter the required units) Hint: Store your calculated value in one of the memories of your calculator (C) with all the decimals to be used in future calculations. Answer: 20.80 kJ/ X Problem 1.5 Constant Volume Process 0.204 kg of air is cooled from 51 C to 17C at a constant volume of 0.03 m. Assume Perfect Gas behavior. Cpair = 29.11 KJ kgmole K Mwair = 28.964 kg kgmole R = 8.3145 KJ kgmole K , K = T(C) + 273.15 Calculate the constant volume specific heat (Cu) in kJ/(kg*K) with the molar specific heat at constant volume and the air's molecular weight. (provide your answer with three decimals and enter the required units) Answer: Check Problem 1.6 Constant Volume Process 0.204 kg of air is cooled from 51 C to 17 C at a constant volume of 0.03 m3. Assume Perfect Gas behavior. Cpair = 29.11 kJ kgmole K Mwair = 28.964 kg kgmole ,R= 8.3145 kJ kgmole K K = T(C) + 273.15 Calculate the internal energy change of the air (AU) in kJ with the calculated specific heat at constant volume. (provide your answer with four significant figures and enter the required units) Hint: Store your calculated value in one of the memories of your calculator (U) with all the decimals to be used in future calculations. Answer: Check Problem 1.7 Constant Volume Process 0.204 kg of air is cooled from 51 C to 17 C at a constant volume of 0.03 m2. Assume Perfect Gas behavior. Cpair = 29.11 k kgmole K Mwair = 28.964 kg kgmole R = 8.3145 kJ kgmole K K = T(C) + 273.15 Calculate the heat (Q) in kj with the calculated internal energy. (provide your answer with four significant figures and enter the required units) Hint: Store your calculated value in one of the memories of your calculator (U) with all the decimals to be used in future calculations. Answer: Check Problem 1.8 Constant Volume Process 0.204 kg of air is cooled from 51 C to 17 C at a constant volume of 0.03 m2. Assume Perfect Gas behavior. Cpair = 29.11 k kgmole K Mwair = 28.964 kg kgmole R = 8.3145 k kgmole K K = T(C) + 273.15 Calculate the work (W) in kJ. (provide your answer with four significant figures and enter the required units) Answer: Check









