Question: The surface of the sun has a temperature of about 5800K and consists largely of hydrogen atoms. Part A Find the rms speed of
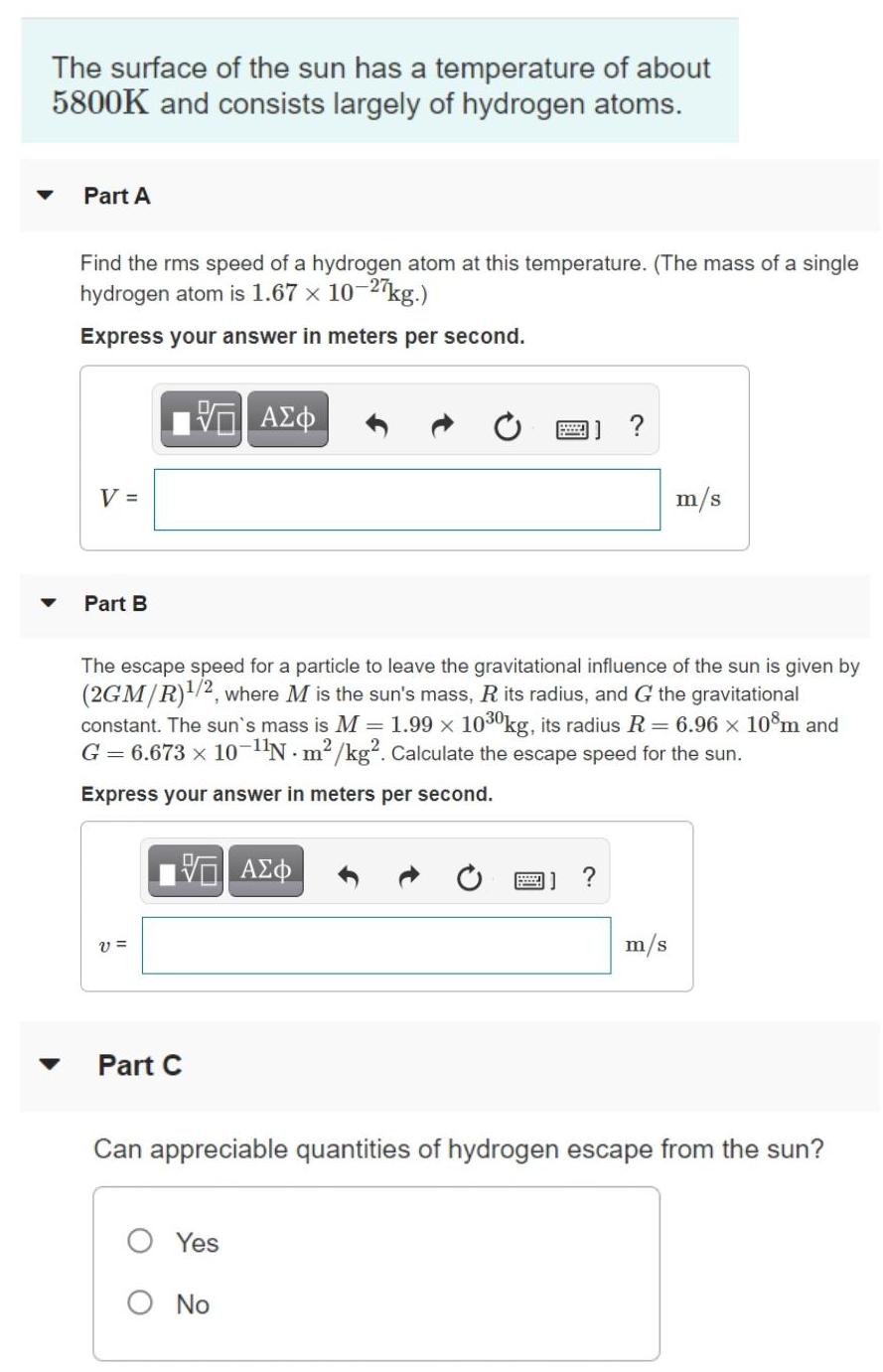
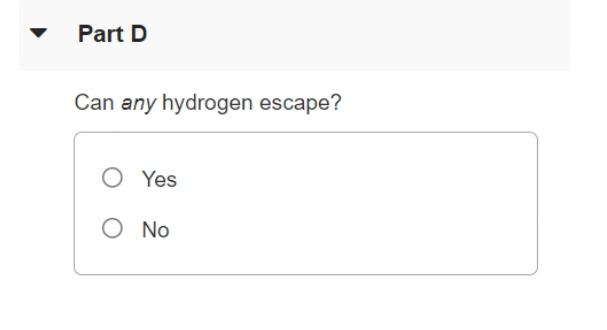
The surface of the sun has a temperature of about 5800K and consists largely of hydrogen atoms. Part A Find the rms speed of a hydrogen atom at this temperature. (The mass of a single hydrogen atom is 1.67 x 10-2 kg.) Express your answer in meters per second. V = m/s Part B The escape speed for a particle to leave the gravitational influence of the sun is given by (2GM/R)/2, where M is the sun's mass, R its radius, and G the gravitational constant. The sun's mass is M = 1.99 x 1030kg, its radius R = 6.96 x 10m and G = 6.673 x 10-N m2 /kg. Calculate the escape speed for the sun. Express your answer in meters per second. A) ? v = m/s Part C Can appreciable quantities of hydrogen escape from the sun? O Yes O No Part D Can any hydrogen escape? O Yes O No
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


