Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 1.One mole of carbon dioxide initially at 30 degree Celsius and 1 bar is first compressed isothermally to 10 bar. Then, heat at constant
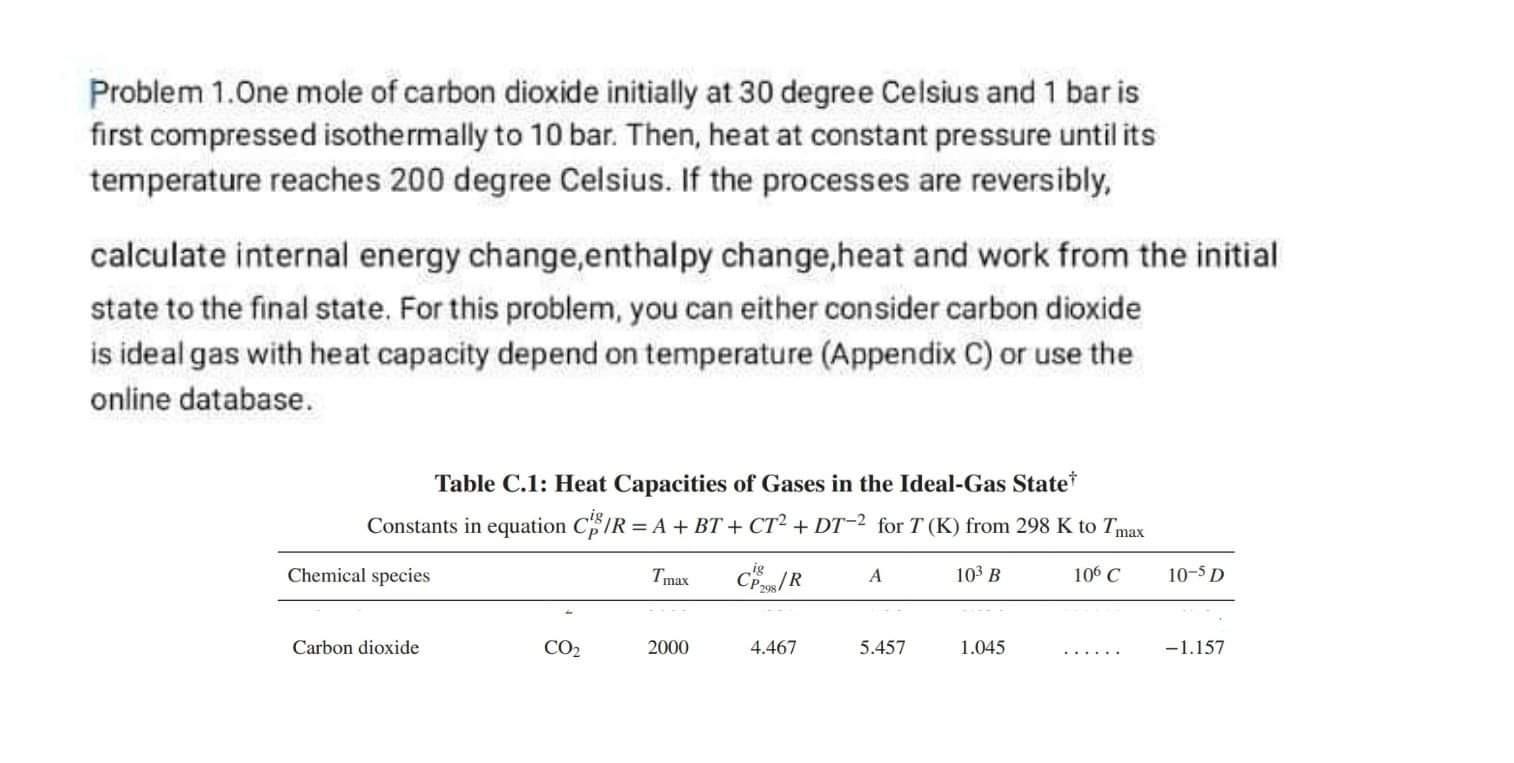
Problem 1.One mole of carbon dioxide initially at 30 degree Celsius and 1 bar is first compressed isothermally to 10 bar. Then, heat at constant pressure until its temperature reaches 200 degree Celsius. If the processes are reversibly, calculate internal energy change,enthalpy change,heat and work from the initial state to the final state. For this problem, you can either consider carbon dioxide is ideal gas with heat capacity depend on temperature (Appendix C) or use the online database. Table C.1: Heat Capacities of Gases in the Ideal-Gas State Constants in equation CPig/R=A+BT+CT2+DT2 for T(K) from 298K to Tmax
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


