Answered step by step
Verified Expert Solution
Question
1 Approved Answer
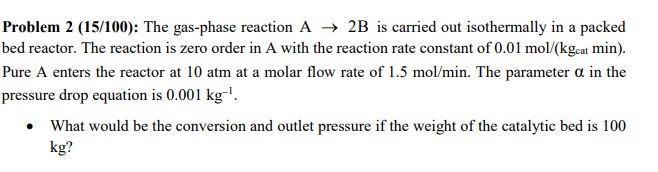
Problem 2 ( 1 5 / 1 0 0 ) : The gas - phase reaction A 2 B is carried out isothermally in a
Problem : The gasphase reaction is carried out isothermally in a packed
bed reactor. The reaction is zero order in A with the reaction rate constant of cat min.
Pure A enters the reactor at atm at a molar flow rate of The parameter in the
pressure drop equation is
What would be the conversion and outlet pressure if the weight of the catalytic bed is
please provide the full solution with answers and steps
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


