Question
Problem #2 (20 points) Three quarts of water at 25 C is poured over 1.5 kilograms of ice that is at -4 C. Assuming
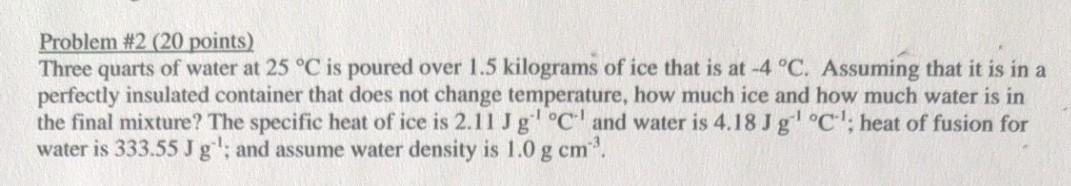
Problem #2 (20 points) Three quarts of water at 25 C is poured over 1.5 kilograms of ice that is at -4 C. Assuming that it is in a perfectly insulated container that does not change temperature, how much ice and how much water is in the final mixture? The specific heat of ice is 2.11 J g C and water is 4.18 Jg C; heat of fusion for water is 333.55 J g'; and assume water density is 1.0 g cm.
Step by Step Solution
3.50 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
Solution m3 quarts 284 kg M15kg T4degree C...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Chemical Engineering Thermodynamics
Authors: J. M. Smith, H. C. Van Ness, M. M. Abbott
7th edition
71247084, 978-0071247085
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



