Question
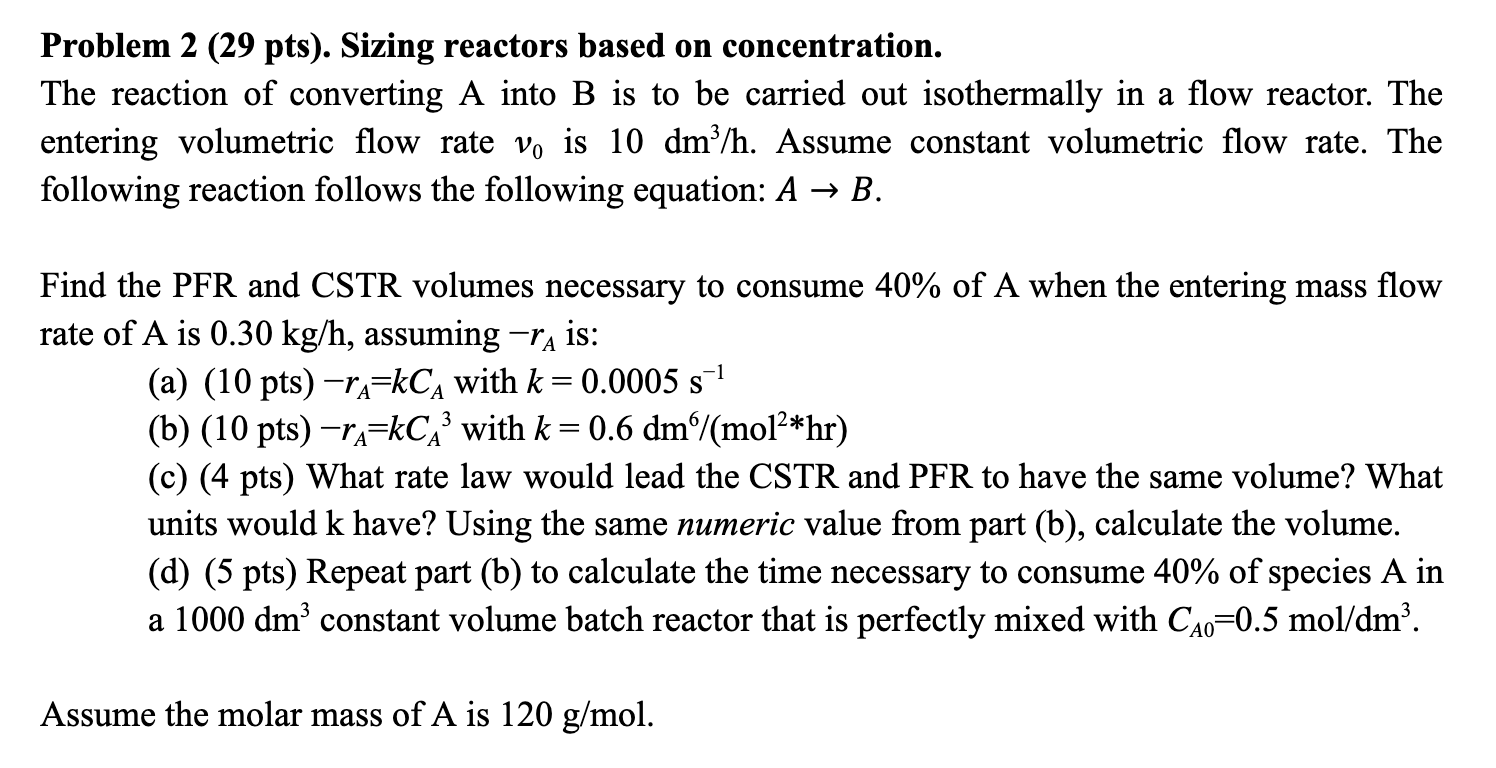
Problem 2 (29 pts). Sizing reactors based on concentration. The reaction of converting A into B is to be carried out isothermally in a flow
Problem 2 (29 pts). Sizing reactors based on concentration.\ The reaction of converting
Ainto
Bis to be carried out isothermally in a flow reactor. The\ entering volumetric flow rate
v_(0)is
10d(m^(3))/(h). Assume constant volumetric flow rate. The\ following reaction follows the following equation:
A->B.\ Find the PFR and CSTR volumes necessary to consume
40%of A when the entering mass flow\ rate of
Ais
0.30k(g)/(h), assuming
-r_(A)is:\ (a)
(10pts)-r_(A)=kC_(A)with
k=0.0005s^(-1)\ (b) (10 pts)
-r_(A)=kC_(A)^(3)with
k=0.6d(m^(6))/(mol^(2**)hr)\ (c) (4 pts) What rate law would lead the CSTR and PFR to have the same volume? What\ units would
khave? Using the same numeric value from part (b), calculate the volume.\ (d) (5 pts) Repeat part (b) to calculate the time necessary to consume
40%of species A in\ a
1000dm^(3)constant volume batch reactor that is perfectly mixed with
C_(A0)=0.5mo(l)/(d)m^(3).\ Assume the molar mass of A is
120(g)/(m)ol.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


