Answered step by step
Verified Expert Solution
Question
1 Approved Answer
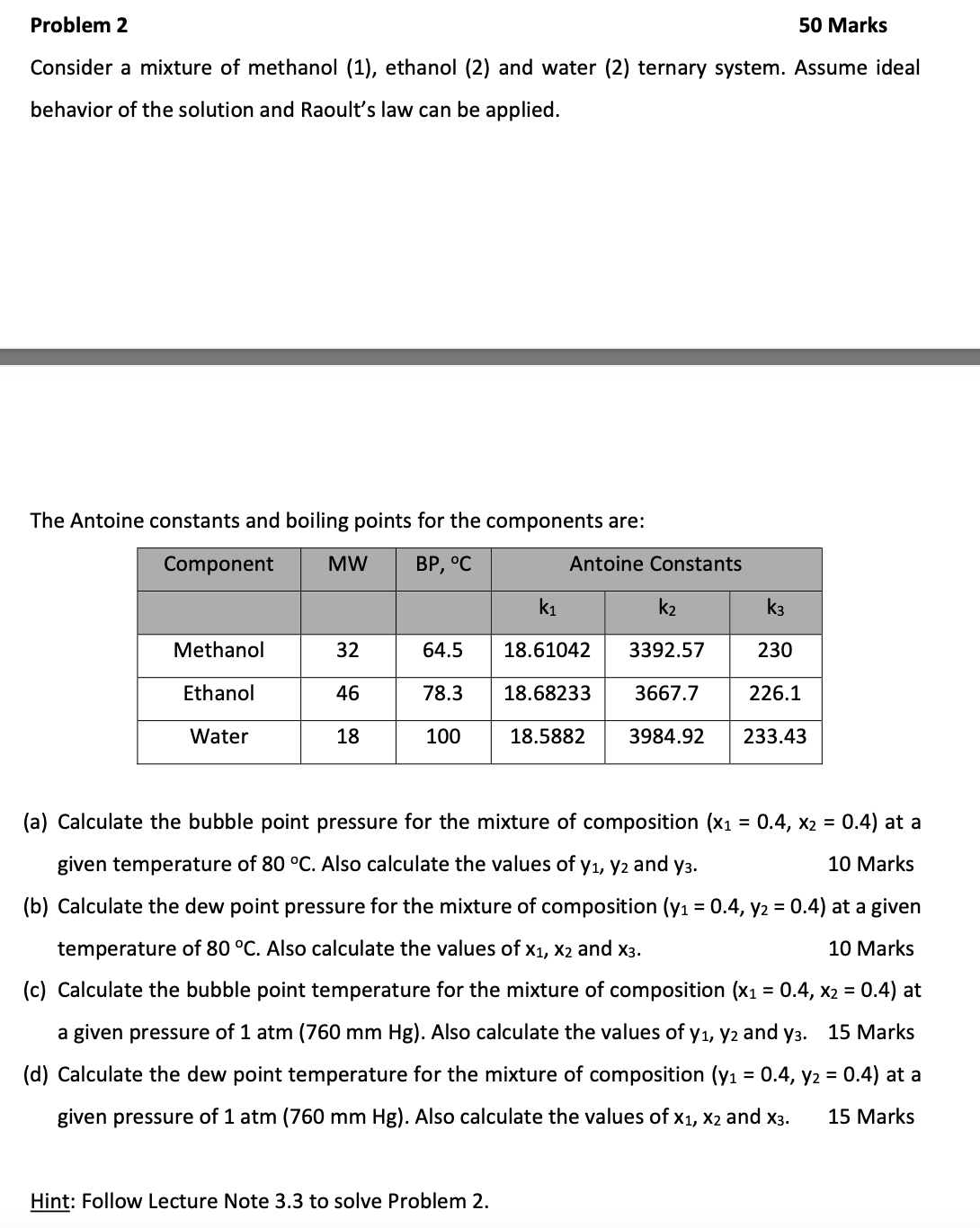
Problem 2 5 0 Marks Consider a mixture of methanol ( 1 ) , ethanol ( 2 ) and water ( 2 ) ternary system.
Problem
Marks
Consider a mixture of methanol ethanol and water ternary system. Assume ideal
behavior of the solution and Raoult's law can be applied.
The Antoine constants and boiling points for the components are:
a Calculate the bubble point pressure for the mixture of composition at a
given temperature of Also calculate the values of and
Marks
b Calculate the dew point pressure for the mixture of composition at a given
temperature of Also calculate the values of and
Marks
c Calculate the bubble point temperature for the mixture of composition at
a given pressure of Also calculate the values of and Marks
d Calculate the dew point temperature for the mixture of composition at a
given pressure of Also calculate the values of and Marks
Hint: Follow Lecture Note to solve Problem Problem Marks
Consider a mixture of methanol ethanol and water ternary system. Assume ideal behavior of the solution and Raoults law can be applied.
Benzene
k
k
k
The Antoine constants and boiling points for the components are:
Component
MW
BP oC
Antoine Constants
k
Methanol
Ethanol
k
k
Water
a Calculate the bubble point pressure for the mixture of composition x x at a given temperature of oC Also calculate the values of y y and y Marks
b Calculate the dew point pressure for the mixture of composition y y at a given temperature of oC Also calculate the values of x x and x Marks
c Calculate the bubble point temperature for the mixture of composition x x at a given pressure of atm mm Hg Also calculate the values of y y and y Marks
d Calculate the dew point temperature for the mixture of composition y y at a given pressure of atm mm Hg Also calculate the values of x x and x Marks
Hint: Follow Lecture Note to solve Problem
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


