Answered step by step
Verified Expert Solution
Question
1 Approved Answer
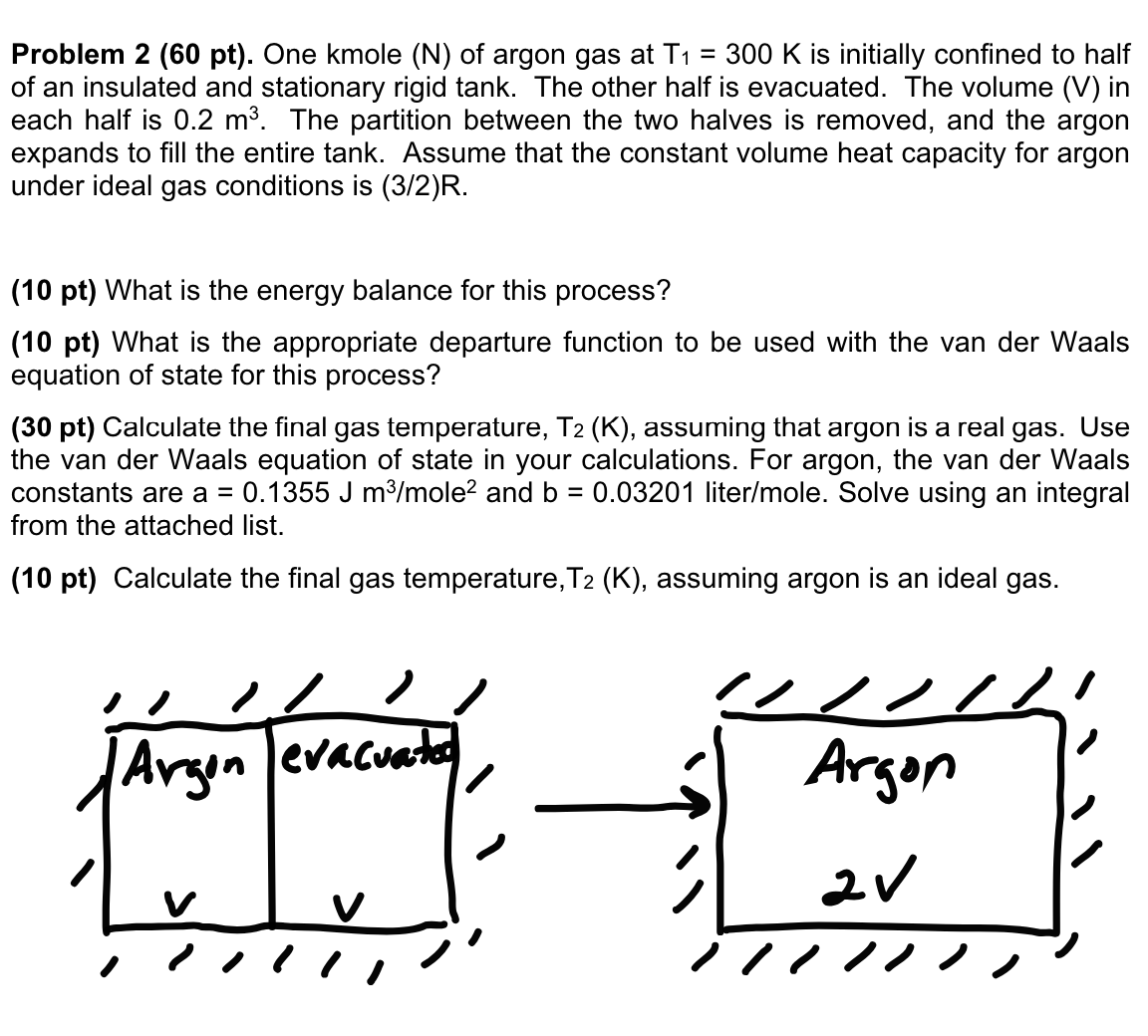
Problem 2 ( 6 0 pt ) . One kmole ( N ) of argon gas at T 1 = 3 0 0 K is
Problem pt One kmole of argon gas at is initially confined to half
of an insulated and stationary rigid tank. The other half is evacuated. The volume V in
each half is The partition between the two halves is removed, and the argon
expands to fill the entire tank. Assume that the constant volume heat capacity for argon
under ideal gas conditions is
pt What is the energy balance for this process?
pt What is the appropriate departure function to be used with the van der Waals
equation of state for this process?
pt Calculate the final gas temperature, assuming that argon is a real gas. Use
the van der Waals equation of state in your calculations. For argon, the van der Waals
constants are and litermole Solve using an integral
from the attached list.
pt Calculate the final gas temperature, assuming argon is an ideal gas.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


