Answered step by step
Verified Expert Solution
Question
1 Approved Answer
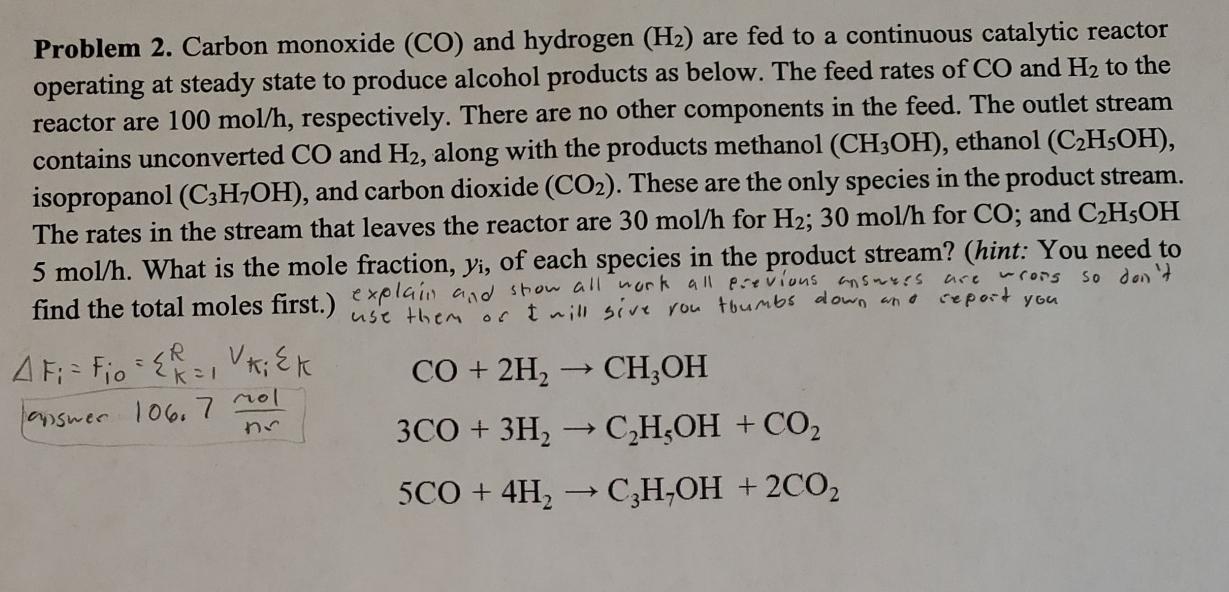
Problem 2 . Carbon monoxide ( C O ) and hydrogen ( H 2 ) are fed to a continuous catalytic reactor operating at steady
Problem Carbon monoxide and hydrogen are fed to a continuous catalytic reactor operating at steady state to produce alcohol products as below. The feed rates of and to the reactor are respectively. There are no other components in the feed. The outlet stream contains unconverted and along with the products methanol ethanol isopropanol and carbon dioxide These are the only species in the product stream. The rates in the stream that leaves the reactor are for ; for ; and What is the mole fraction, of each species in the product stream? hint: You need to find the total moles first. explaii and stow all work all previous answers are wrors so don't use them of aill sive rou toumbs down and seport you aswer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


