Answered step by step
Verified Expert Solution
Question
1 Approved Answer
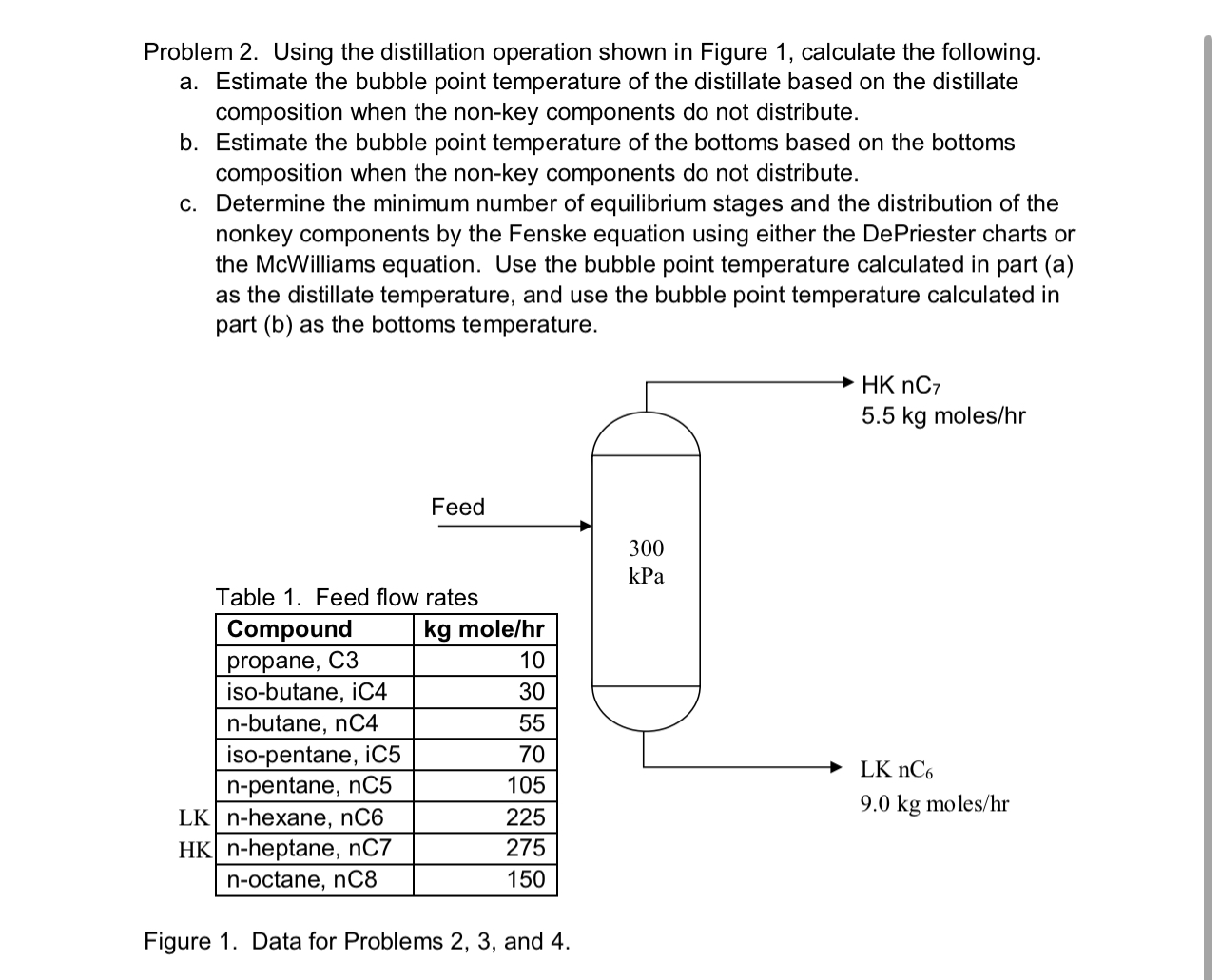
Problem 2 . Using the distillation operation shown in Figure 1 , calculate the following. a . Estimate the bubble point temperature of the distillate
Problem Using the distillation operation shown in Figure calculate the following.
a Estimate the bubble point temperature of the distillate based on the distillate composition when the nonkey components do not distribute.
b Estimate the bubble point temperature of the bottoms based on the bottoms composition when the nonkey components do not distribute.
c Determine the minimum number of equilibrium stages and the distribution of the nonkey components by the Fenske equation using either the DePriester charts or the McWilliams equation. Use the bubble point temperature calculated in part a as the distillate temperature, and use the bubble point temperature calculated in part b as the bottoms temperature.
Feed
Table Feed flow rates
tableCompoundkgmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


