Question
Problem 2: You want to produce TiO2 nanoparticles with a TiCl4-fed flame reactor. The burning flame has a measured temperature of 2000C. a) Assuming
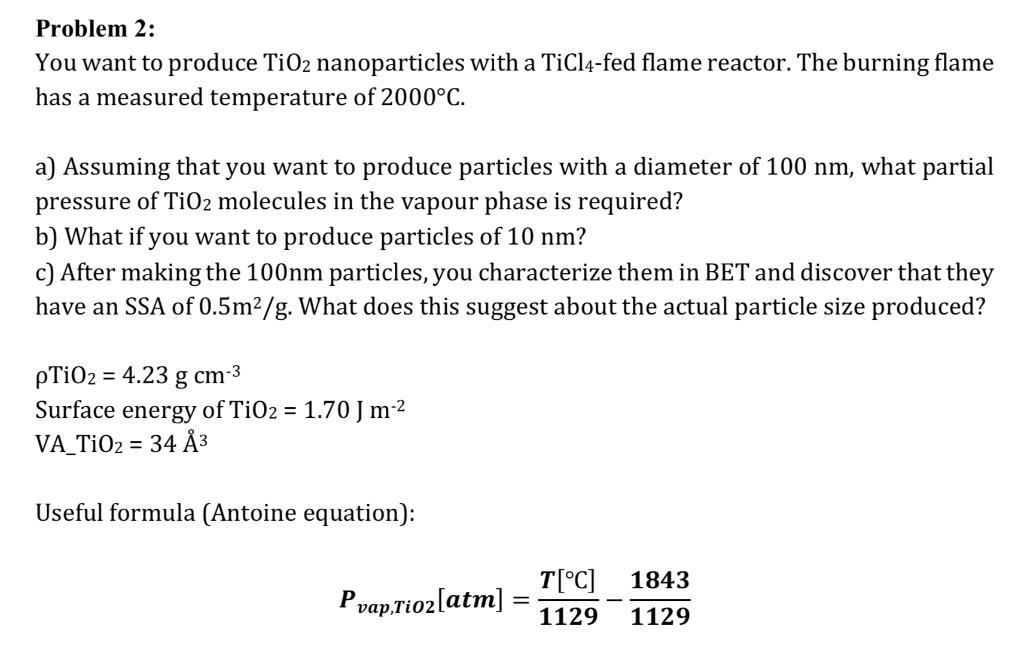
Problem 2: You want to produce TiO2 nanoparticles with a TiCl4-fed flame reactor. The burning flame has a measured temperature of 2000C. a) Assuming that you want to produce particles with a diameter of 100 nm, what partial pressure of TiO2 molecules in the vapour phase is required? b) What if you want to produce particles of 10 nm? c) After making the 100nm particles, you characterize them in BET and discover that they have an SSA of 0.5m/g. What does this suggest about the actual particle size produced? pTiO2 = 4.23 g cm- Surface energy of TiO2 = 1.70 J m- VA_TiO = 34 Useful formula (Antoine equation): Pvap,Tio2[atm] = T[C] 1843 1129 1129
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
a the partial pressure of tig melecules in the vapeur phase ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: James S. Walker
5th edition
978-0133498493, 9780321909107, 133498492, 0321909100, 978-0321976444
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



