Question
Problem 29-2E. Waste water containing the dissolved contaminant naphthalene is sent to a well-mixed open pond, as shown in Figure 29-2Ea. The pond allows
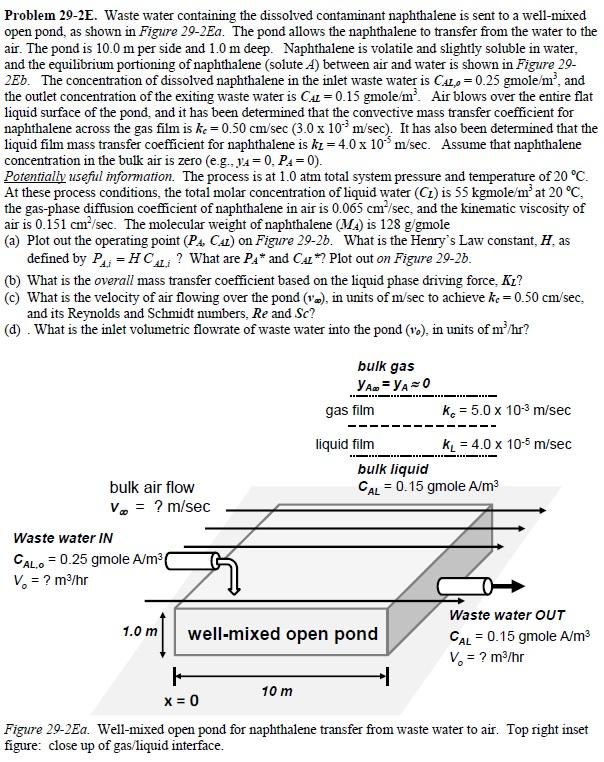
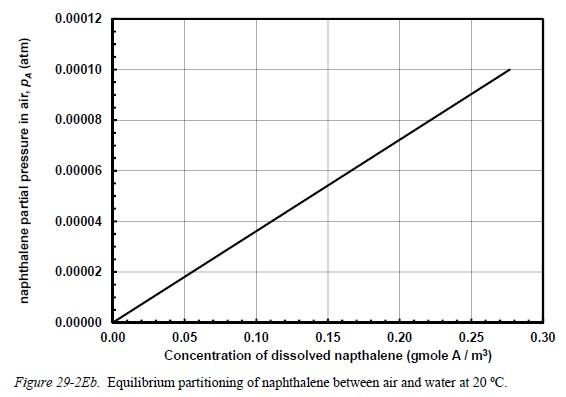
Problem 29-2E. Waste water containing the dissolved contaminant naphthalene is sent to a well-mixed open pond, as shown in Figure 29-2Ea. The pond allows the naphthalene to transfer from the water to the air. The pond is 10.0 m per side and 1.0 m deep. Naphthalene is volatile and slightly soluble in water, and the equilibrium portioning of naphthalene (solute 4) between air and water is shown in Figure 29- 2Eb. The concentration of dissolved naphthalene in the inlet waste water is CAL = 0.25 gmole/m, and the outlet concentration of the exiting waste water is CAL = 0.15 gmole/m. Air blows over the entire flat liquid surface of the pond, and it has been determined that the convective mass transfer coefficient for naphthalene across the gas film is ke = 0.50 cm/sec (3.0 x 103 m/sec). It has also been determined that the liquid film mass transfer coefficient for naphthalene is kz = 4.0 x 105 m/sec. Assume that naphthalene concentration in the bulk air is zero (e.g., y = 0, PA=0). Potentially useful information. The process is at 1.0 atm total system pressure and temperature of 20 C. At these process conditions, the total molar concentration of liquid water (Cz) is 55 kgmole/m at 20 C, the gas-phase diffusion coefficient of naphthalene in air is 0.065 cm/sec, and the kinematic viscosity of air is 0.151 cm/sec. The molecular weight of naphthalene (MA) is 128 g/gmole (a) Plot out the operating point (PA, CA) on Figure 29-2b. What is the Henry's Law constant, H. as defined by P = HCAL? What are PA and CAL*? Plot out on Figure 29-2b. (b) What is the overall mass transfer coefficient based on the liquid phase driving force, KI? (c) What is the velocity of air flowing over the pond (v), in units of m/sec to achieve kc = 0.50 cm/sec, and its Reynolds and Schmidt numbers, Re and Sc? (d). What is the inlet volumetric flowrate of waste water into the pond (v.), in units of m/hr? bulk air flow V = ? m/sec Waste water IN CALO 0.25 gmole A/m ( V = ? m/hr bulk gas YA = YA=0 gas film ke 5.0 x 10-3 m/sec liquid film k = 4.0 x 10-5 m/sec bulk liquid CAL = 0.15 gmole A/m 1.0 m well-mixed open pond x=0 10 m + Waste water OUT CAL 0.15 gmole A/m V = ? m/hr Figure 29-2Ea. Well-mixed open pond for naphthalene transfer from waste water to air. Top right inset figure: close up of gas/liquid interface. naphthalene partial pressure in air, PA (atm) 0.00012 0.00010 0.00008 0.00006 0.00004 0.00002 0.00000 0.00 0.15 0.20 0.05 0.10 0.25 Concentration of dissolved napthalene (gmole A/m) Figure 29-2Eb. Equilibrium partitioning of naphthalene between air and water at 20 C. 0.30
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access with AI-Powered Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


