Answered step by step
Verified Expert Solution
Question
1 Approved Answer
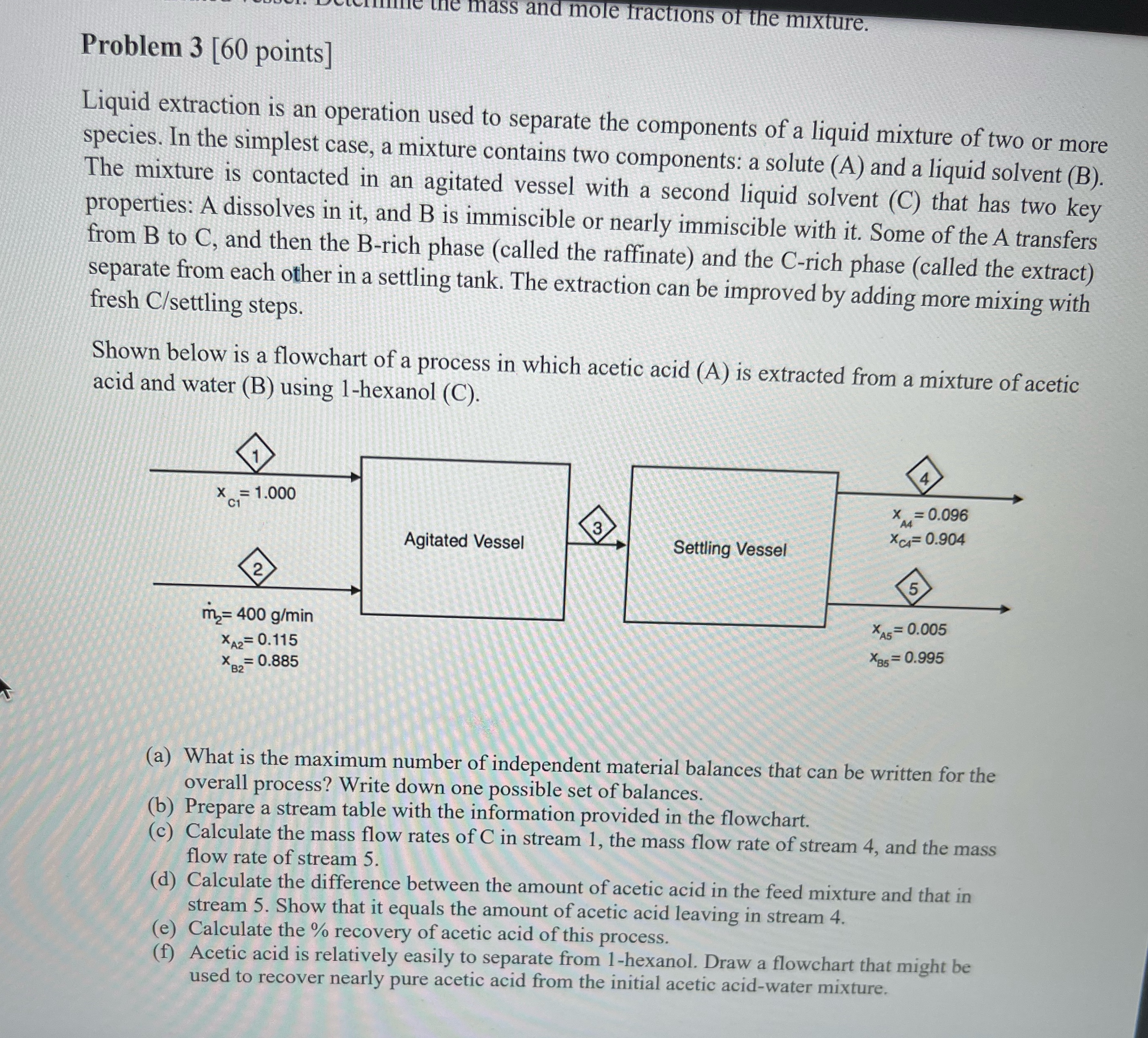
Problem 3 [ 6 0 points ] Liquid extraction is an operation used to separate the components of a liquid mixture of two or more
Problem points
Liquid extraction is an operation used to separate the components of a liquid mixture of two or more species. In the simplest case, a mixture contains two components: a solute A and a liquid solvent B The mixture is contacted in an agitated vessel with a second liquid solvent C that has two key properties: A dissolves in it and B is immiscible or nearly immiscible with it Some of the A transfers from to and then the Brich phase called the raffinate and the Crich phase called the extract separate from each other in a settling tank. The extraction can be improved by adding more mixing with fresh settling steps.
Shown below is a flowchart of a process in which acetic acid A is extracted from a mixture of acetic acid and water B using hexanol C
a What is the maximum number of independent material balances that can be written for the overall process? Write down one possible set of balances.
b Prepare a stream table with the information provided in the flowchart.
c Calculate the mass flow rates of in stream the mass flow rate of stream and the mass flow rate of stream
d Calculate the difference between the amount of acetic acid in the feed mixture and that in stream Show that it equals the amount of acetic acid leaving in stream
e Calculate the recovery of acetic acid of this process.
f Acetic acid is relatively easily to separate from hexanol. Draw a flowchart that might be used to recover nearly pure acetic acid from the initial acetic acidwater mixture.S
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


