Answered step by step
Verified Expert Solution
Question
1 Approved Answer
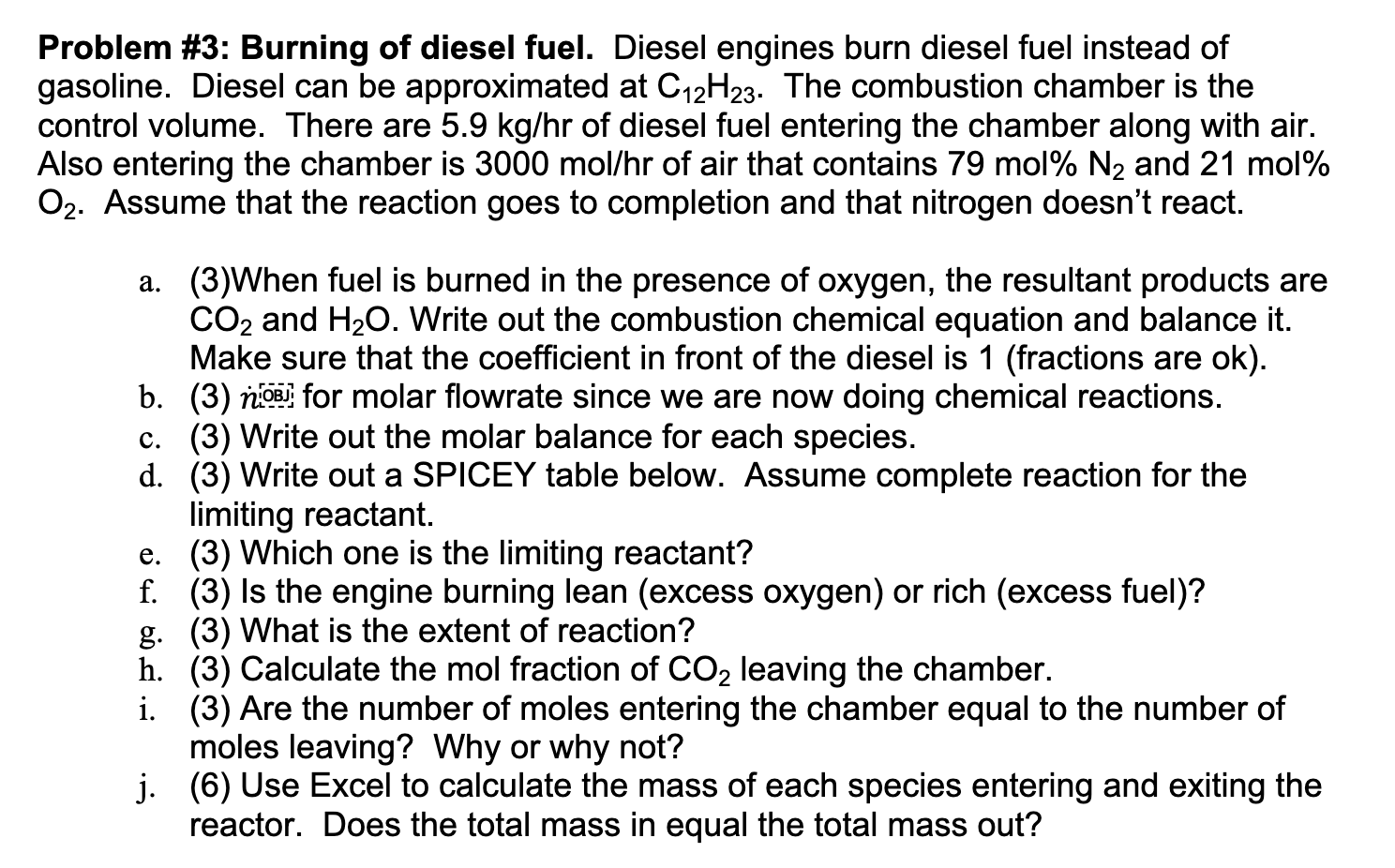
Problem # 3 : Burning of diesel fuel. Diesel engines burn diesel fuel instead of gasoline. Diesel can be approximated at C 1 2 H
Problem #: Burning of diesel fuel. Diesel engines burn diesel fuel instead of
gasoline. Diesel can be approximated at The combustion chamber is the
control volume. There are of diesel fuel entering the chamber along with air.
Also entering the chamber is of air that contains mol and mol
Assume that the reaction goes to completion and that nitrogen doesn't react.
aWhen fuel is burned in the presence of oxygen, the resultant products are
and Write out the combustion chemical equation and balance it
Make sure that the coefficient in front of the diesel is fractions are ok
b for molar flowrate since we are now doing chemical reactions.
c Write out the molar balance for each species.
d Write out a SPICEY table below. Assume complete reaction for the
limiting reactant.
e Which one is the limiting reactant?
f Is the engine burning lean excess oxygen or rich excess fuel
g What is the extent of reaction?
h Calculate the mol fraction of leaving the chamber.
i Are the number of moles entering the chamber equal to the number of
moles leaving? Why or why not?
j Use Excel to calculate the mass of each species entering and exiting the
reactor. Does the total mass in equal the total mass out?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


