Answered step by step
Verified Expert Solution
Question
1 Approved Answer
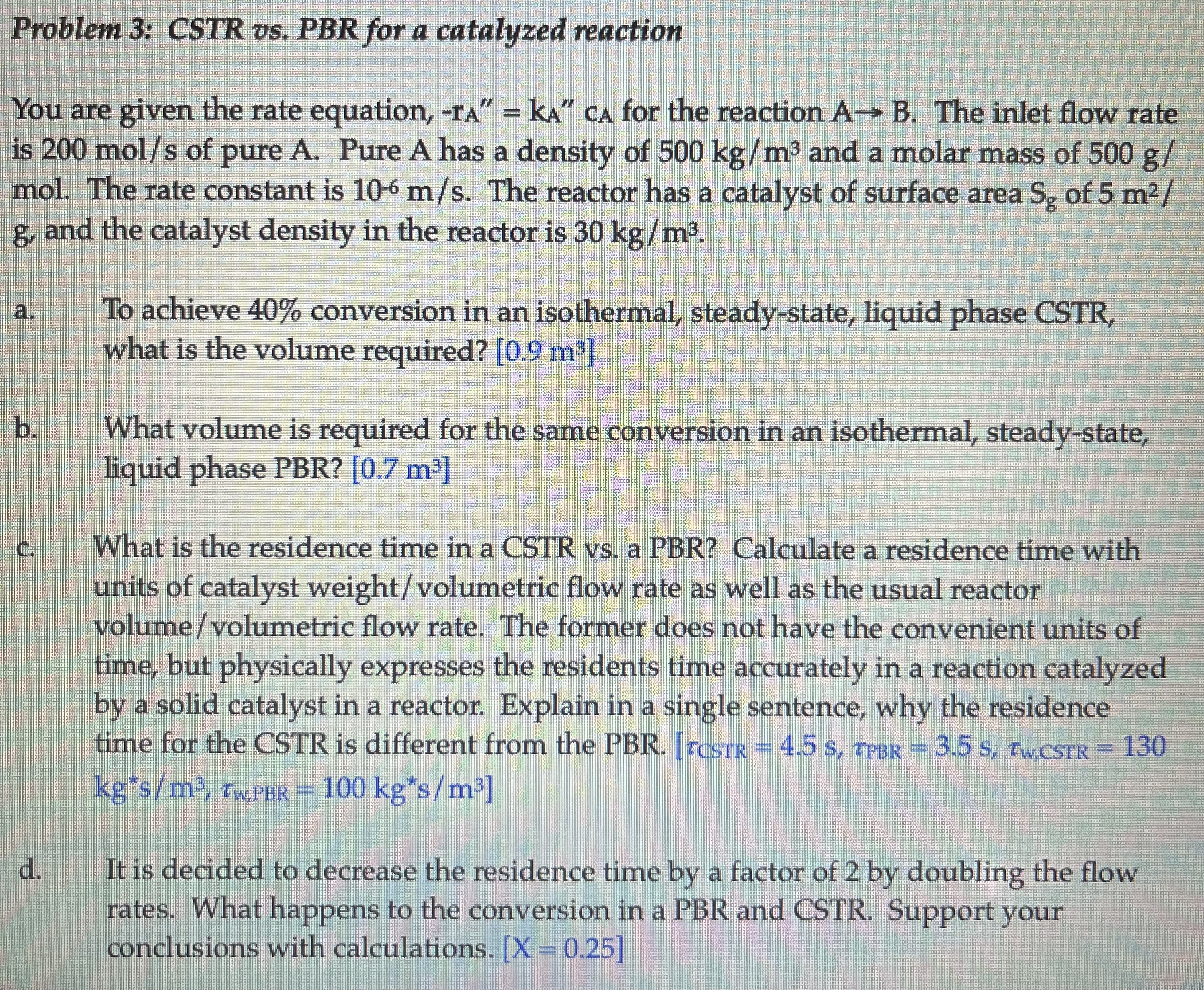
Problem 3 : CSTR vs . PBR for a catalyzed reaction You are given the rate equation, - rA = kA cA for the reaction
Problem : CSTR vs PBR for a catalyzed reaction
You are given the rate equation, rA kA cA for the reaction A B The inlet flow rate
is mols of pure A Pure A has a density of kgm and a molar mass of g
mol. The rate constant is ms The reactor has a catalyst of surface area Sg of m
g and the catalyst density in the reactor is kgm
a To achieve conversion in an isothermal, steadystate, liquid phase CSTR
what is the volume required? m
b What volume is required for the same conversion in an isothermal, steadystate,
liquid phase PBR m
c What is the residence time in a CSTR vs a PBR Calculate a residence time with
units of catalyst weightvolumetric flow rate as well as the usual reactor
volumevolumetric flow rate. The former does not have the convenient units of
time, but physically expresses the residents time accurately in a reaction catalyzed
by a solid catalyst in a reactor. Explain in a single sentence, why the residence
time for the CSTR is different from the PBRCSTR sPBR swCSTR
kgsmwPBR kgsm
d It is decided to decrease the residence time by a factor of by doubling the flow
rates. What happens to the conversion in a PBR and CSTR Support your
conclusions with calculations. X
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


