Answered step by step
Verified Expert Solution
Question
1 Approved Answer
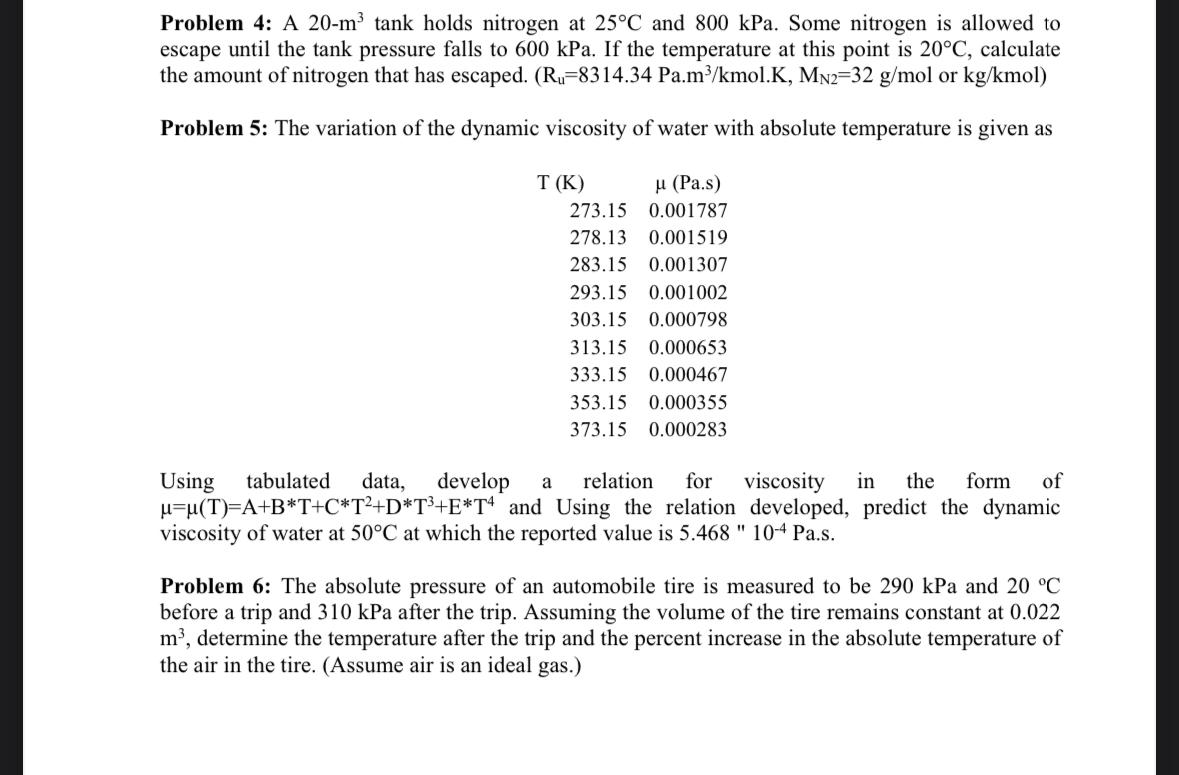
Problem 4 : A 2 0 - m 3 tank holds nitrogen at 2 5 C and 8 0 0 kPa. Some nitrogen is allowed
Problem : A tank holds nitrogen at and kPa. Some nitrogen is allowed to escape until the tank pressure falls to kPa. If the temperature at this point is calculate the amount of nitrogen that has escaped. or :mol
Problem : The variation of the dynamic viscosity of water with absolute temperature is given as
tablePas
Using tabulated data, develop a relation for viscosity in the form of and Using the relation developed, predict the dynamic viscosity of water at at which the reported value is s
Problem : The absolute pressure of an automobile tire is measured to be kPa and before a trip and kPa after the trip. Assuming the volume of the tire remains constant at determine the temperature after the trip and the percent increase in the absolute temperature of the air in the tire. Assume air is an ideal gas.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


