Answered step by step
Verified Expert Solution
Question
1 Approved Answer
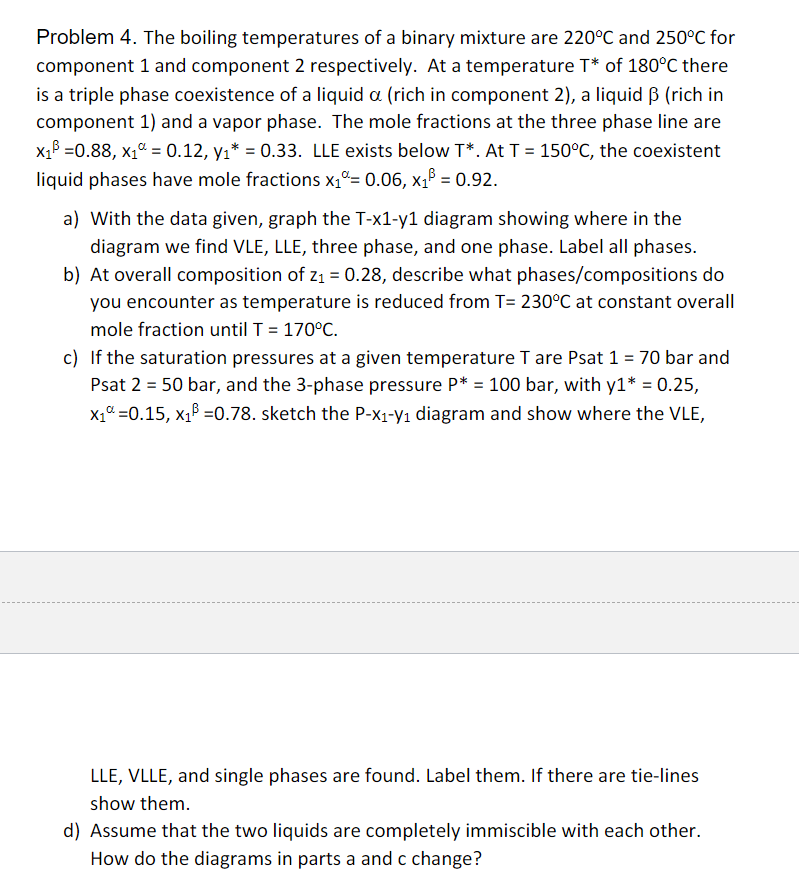
Problem 4 . The boiling temperatures of a binary mixture are 2 2 0 C and 2 5 0 C for component 1 and component
Problem The boiling temperatures of a binary mixture are and for
component and component respectively. At a temperature of there
is a triple phase coexistence of a liquid rich in component a liquid rich in
component and a vapor phase. The mole fractions at the three phase line are
LLE exists below At the coexistent
liquid phases have mole fractions
a With the data given, graph the Txy diagram showing where in the
diagram we find VLE, LLE, three phase, and one phase. Label all phases.
b At overall composition of describe what phasescompositions do
you encounter as temperature is reduced from at constant overall
mole fraction until
c If the saturation pressures at a given temperature are Psat bar and
Psat bar, and the phase pressure bar, with
sketch the diagram and show where the VLE,
LLE, VLLE, and single phases are found. Label them. If there are tielines
show them.
d Assume that the two liquids are completely immiscible with each other.
How do the diagrams in parts a and c change?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


