Answered step by step
Verified Expert Solution
Question
1 Approved Answer
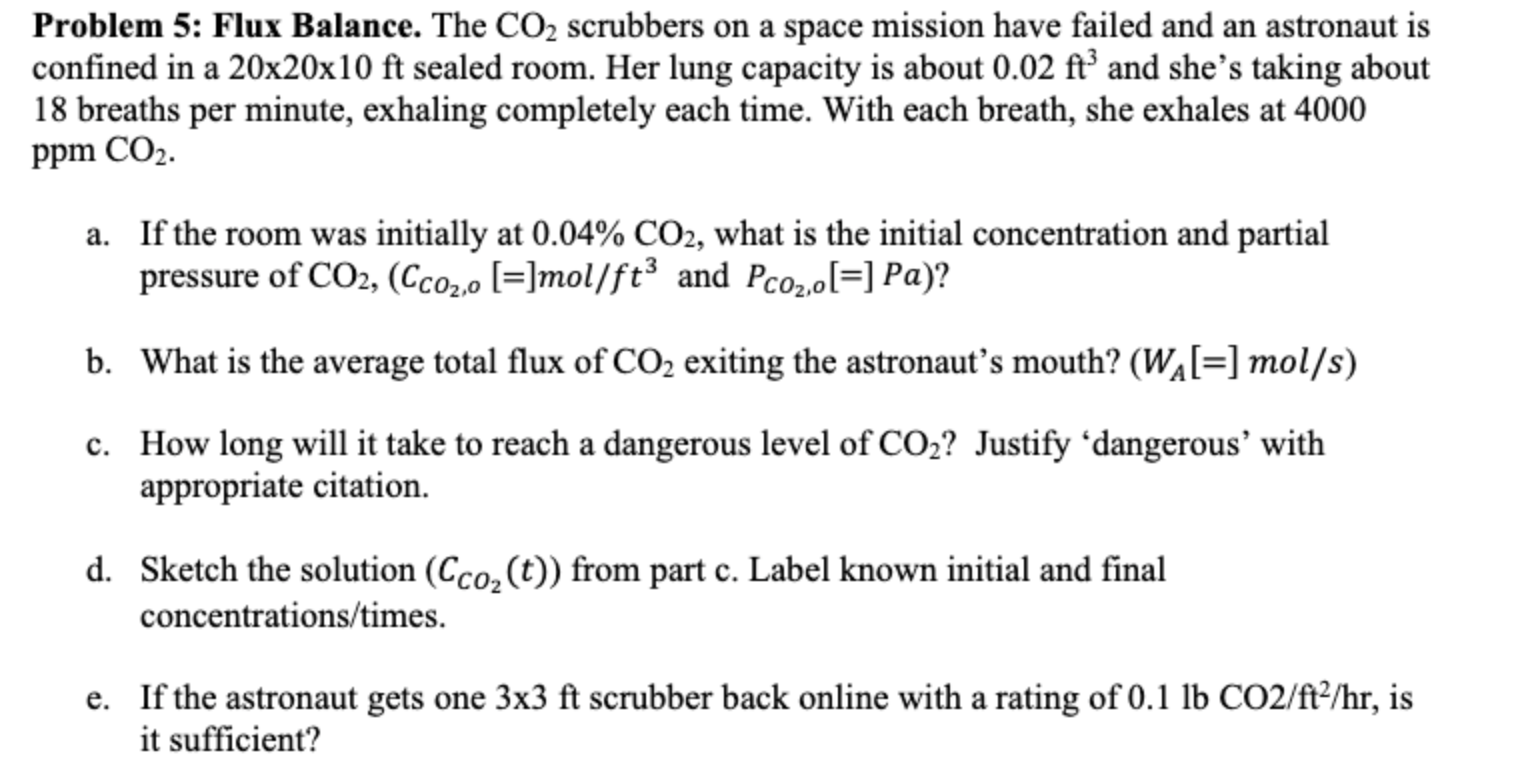
Problem 5 : Flux Balance. The CO 2 scrubbers on a space mission have failed and an astronaut is confined in a 2 0
Problem : Flux Balance. The CO scrubbers on a space mission have failed and an astronaut is
confined in a times times ft sealed room. Her lung capacity is about ft and she's taking about
breaths per minute, exhaling completely each time. With each breath, she exhales at
ppm COz.
a If the room was initially at CO what is the initial concentration and partial
pressure of COCcomolft and PcozolPa
b What is the average total flux of COz exiting the astronaut's mouth? WA mols
c How long will it take to reach a dangerous level of CO Justify dangerous' with
appropriate citation.
d Sketch the solution Ccot from part c Label known initial and final
concentrationstimes
e If the astronaut gets one x ft scrubber back online with a rating of lb COfthr is
it sufficient
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


