Question
Part A Determine whether each of the following salts will form a solution that is acidic, basic, or pH-neutral. Drag the appropriate items to
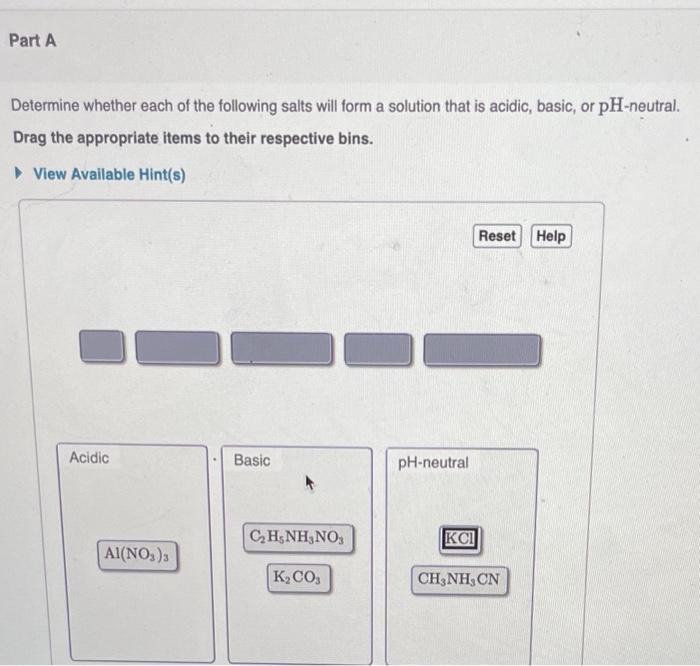
Part A Determine whether each of the following salts will form a solution that is acidic, basic, or pH-neutral. Drag the appropriate items to their respective bins. View Available Hint(s) Acidic Al(NO3)3 Basic CH5NHNO3 KCO3 pH-neutral KCI Reset Help CHNH CN
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
The following salts form following solution ACIDIC BASIC pH NEUTRAL AlNO 3 3 K 2 CO 3 KCl C 2 H 5 NH ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Mathematics for Business Economics Life Sciences and Social Sciences
Authors: Raymond A. Barnett, Michael R. Ziegler, Karl E. Byleen
12th edition
321614003, 978-0321614001
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



