Question: PROBLEM 6. (20 points) Assume a solution of boron and water. Density of water is 1.0 g/cm and the concentration of natural boron is
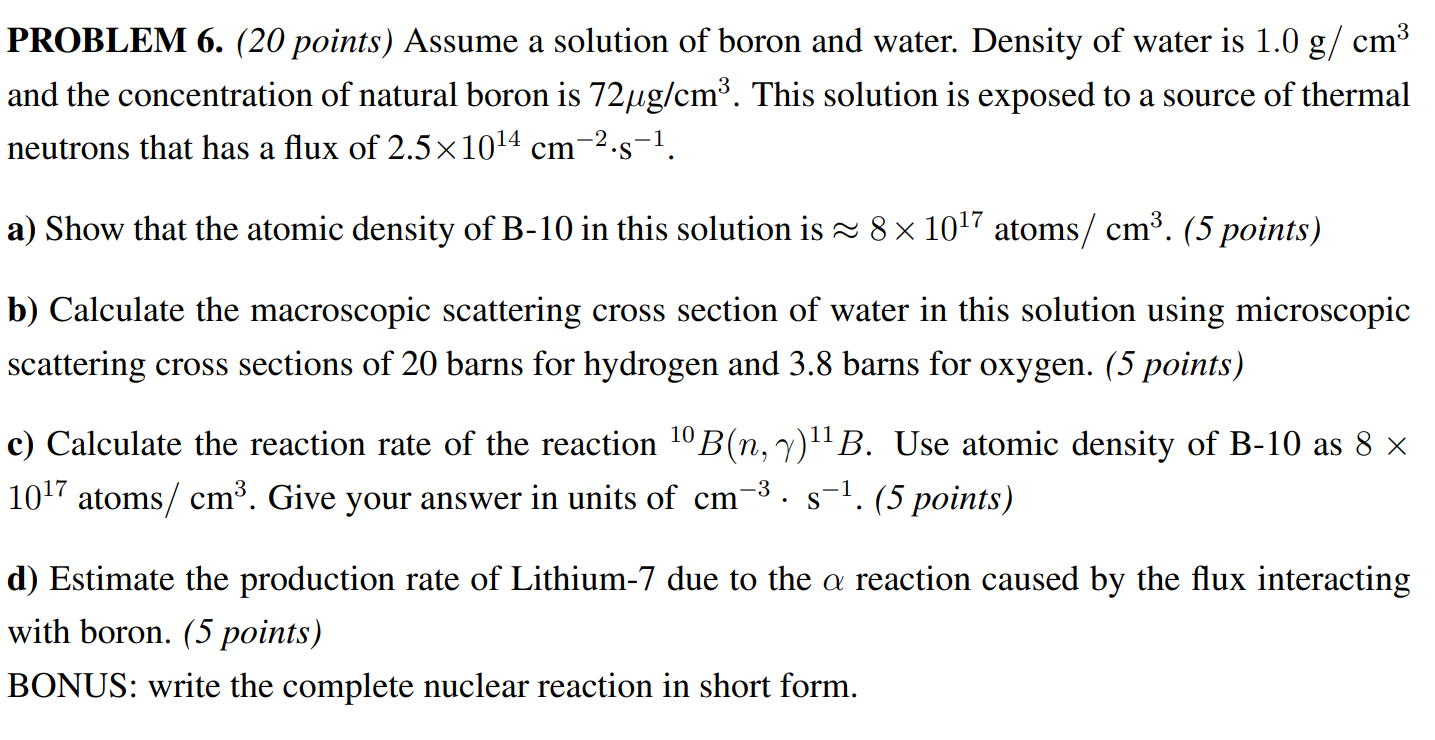
PROBLEM 6. (20 points) Assume a solution of boron and water. Density of water is 1.0 g/cm and the concentration of natural boron is 72g/cm. This solution is exposed to a source of thermal neutrons that has a flux of 2.51014 cm s. -2 a) Show that the atomic density of B-10 in this solution is 8 1017 atoms/ cm. (5 points) b) Calculate the macroscopic scattering cross section of water in this solution using microscopic scattering cross sections of 20 barns for hydrogen and 3.8 barns for oxygen. (5 points) c) Calculate the reaction rate of the reaction 10 B(n, Y) 11 B. Use atomic density of B-10 as 8 1017 atoms/ cm. Give your answer in units of cm s-1. (5 points) d) Estimate the production rate of Lithium-7 due to the a reaction caused by the flux interacting with boron. (5 points) BONUS: write the complete nuclear reaction in short form.
Step by Step Solution
There are 3 Steps involved in it
Understanding the Problem We have problem with a solution of boron in water that is exposed to a neutron flux Solution a Atomic Density of B10 Given C... View full answer

Get step-by-step solutions from verified subject matter experts


