Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 7. BrCH2CH2Br (DBE) contaminated an aquifer which is a drinking water supply at a concentration of 200g/L. The DBE is transformed to HOCH2CH2OH(75%) and
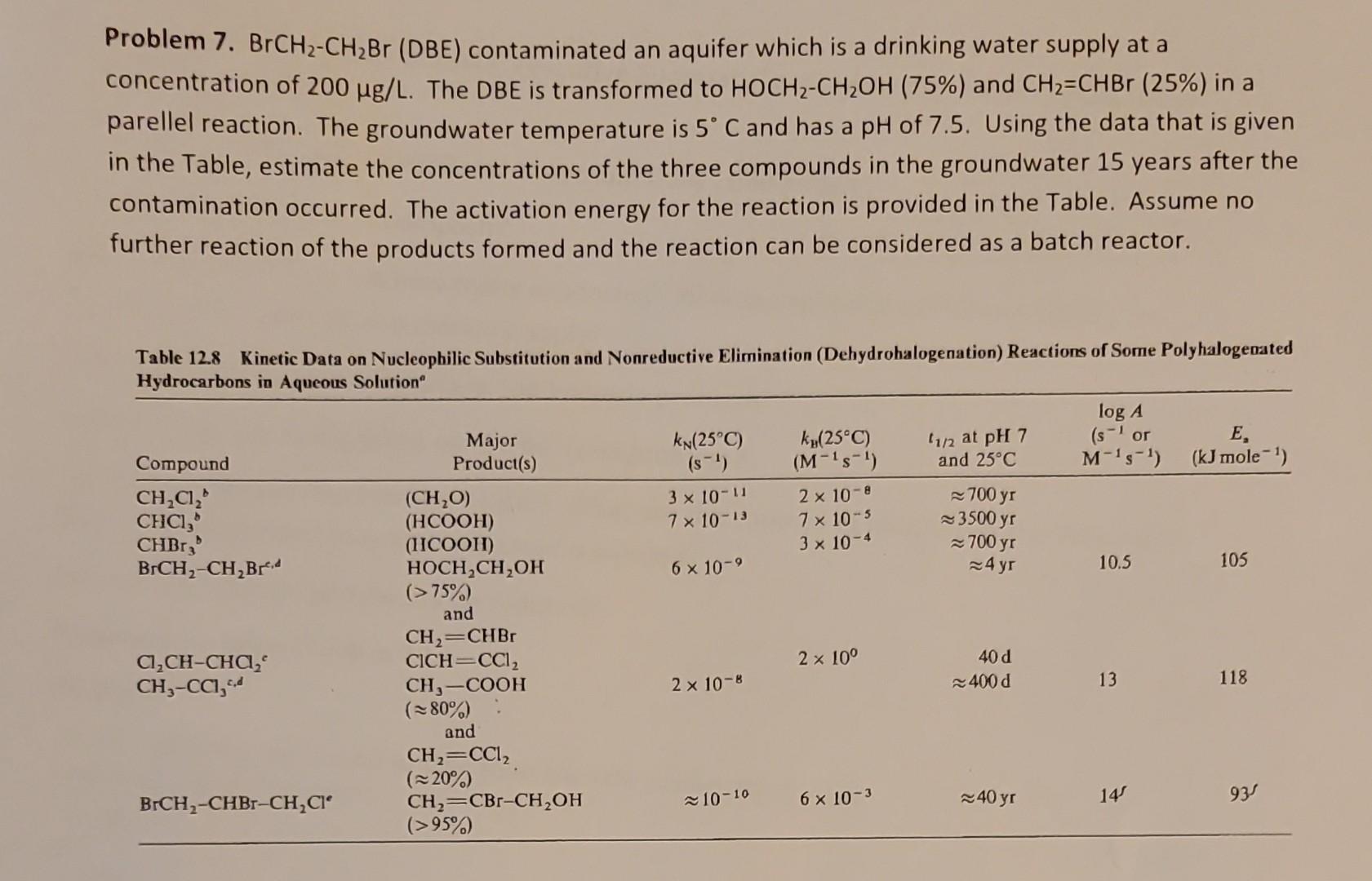
Problem 7. BrCH2CH2Br (DBE) contaminated an aquifer which is a drinking water supply at a concentration of 200g/L. The DBE is transformed to HOCH2CH2OH(75%) and CH2=CHBr(25%) in a parellel reaction. The groundwater temperature is 5C and has a pH of 7.5. Using the data that is given in the Table, estimate the concentrations of the three compounds in the ground 15 years after the contamination occurred. The activation energy for the reaction is provided in the Table. Assume no further reaction of the products formed and the reaction can be considered as a batch reactor. Table 12.8 Kinetic Data on Nucleophilic Substitution and Nonreductive Elimination (Dehydrohalogenation) Reactions of Some Polyhalogenated Hydrocarbons in Aqueous Solution
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


