Answered step by step
Verified Expert Solution
Question
1 Approved Answer
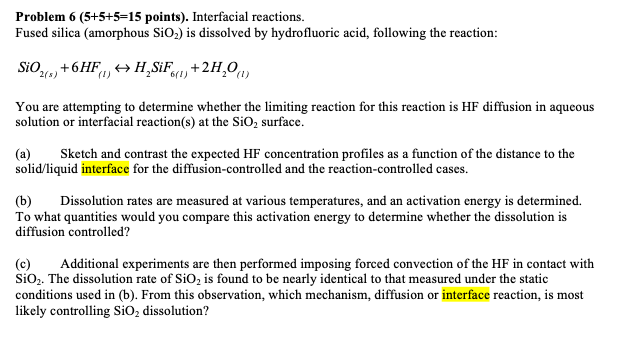
Problem points ) . Interfacial reactions. Fused silica ( amorphous S i O 2 ) is dissolved by hydrofluoric acid, following the reaction: S i
Problem points Interfacial reactions.
Fused silica amorphous is dissolved by hydrofluoric acid, following the reaction:
You are attempting to determine whether the limiting reaction for this reaction is HF diffusion in aqueous
solution or interfacial reactions at the surface.
a Sketch and contrast the expected concentration profiles as a function of the distance to the
solidliquid interface for the diffusioncontrolled and the reactioncontrolled cases.
b Dissolution rates are measured at various temperatures, and an activation energy is determined.
To what quantities would you compare this activation energy to determine whether the dissolution is
diffusion controlled?
c Additional experiments are then performed imposing forced convection of the HF in contact with
The dissolution rate of is found to be nearly identical to that measured under the static
conditions used in b From this observation, which mechanism, diffusion or interface reaction, is most
likely controlling dissolution?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


