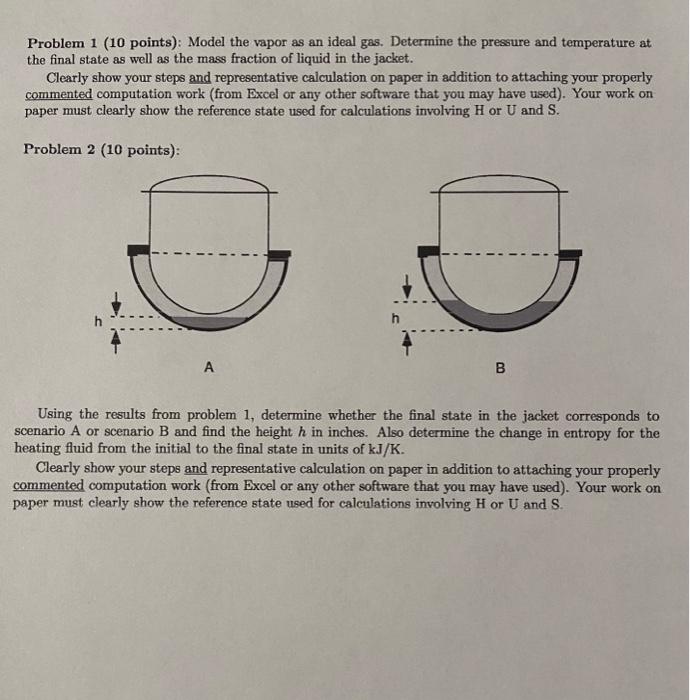
Problem Statement: A chemical company PolyRUs Inc. plans to use a batch reactor for polymerization. To supply energy for the endothermic reaction, a heating jacket is to be used. Since the reaction is sensitive to water, steam cannot be used to supply heating in the jacket Therefore, a pure substance X is used as the heating fluid. The heating fluid is enclosed by rigid walls and heat flows across only the inside surface common to the reactor and heating jacket. The outside of the jacket is insulated so no hent is lost to the surroundings. inside radius of the hemispherical bottom of the reactor R, is 17.5 ft and the gap between the jacket walls, where the heating fluid resides, is 2 inch. Reactor R1 R2 insulation Heating jacket kit For a design case, the heating fluid in vapor phase is pumped into the jacket and inflow/outflow valves closed when the temperature reaches 500C and the pressure rcachos 41.5 bar. The polymerization caction is initiated and heat is supplied from the jacket; when polymerization is quenched after 80 minutes, it is estimated that 200 MJ of energy has been transferred from the jacket. At the final condition, it is found that there is liquid condensate in the jacket along with vapor. Data given for the substance X with MW = 45: Specific heat in vapor phase: J CE mol. K ) = 25.2 +0.002T +1.3 x 10-5 - 3 x 10-9 where T is in K Antoine equation: 1830.6 log10 P = 7.5 - 225.1+T where P is in mmHg and T is in C Density of liquid p(kg/m) = 867 -0.217 +2.66 x 10 T - 1.02 x 10-73 where T is in C for phase change enthalpy use the relation given in section 2.10 of the textbook (equation 2.46) 2 Problem 1 (10 points): Model the vapor as an ideal gas. Determine the pressure and temperature at the final state as well as the mass fraction of liquid in the jacket. Clearly show your steps and representative calculation on paper in addition to attaching your properly commented computation work (from Excel or any other software that you may have used). Your work on paper must clearly show the reference state used for calculations involving H or U and S. Problem 2 (10 points): A B Using the results from problem 1, determine whether the final state in the jacket corresponds to scenario A or scenario B and find the height h in inches. Also determine the change in entropy for the heating fluid from the initial to the final state in units of kJ/K. Clearly show your steps and representative calculation on paper in addition to attaching your properly commented computation work (from Excel or any other software that you may have used). Your work on paper must clearly show the reference state used for calculations involving H or U and S