Answered step by step
Verified Expert Solution
Question
1 Approved Answer
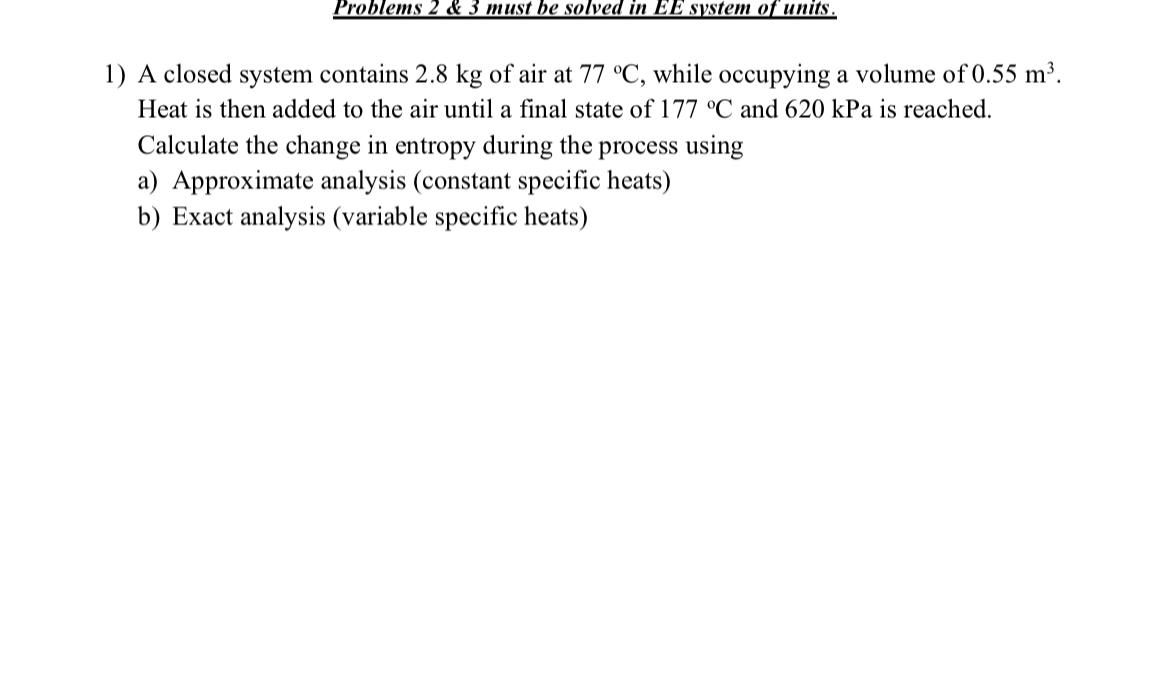
Problems 2 & 3 must be solved in EE system of units. A closed system contains 2.8kg of air at 77deg C , while occupying
Problems 2 & 3 must be solved in EE system of units.\ A closed system contains
2.8kgof air at
77\\\\deg C, while occupying a volume of
0.55m^(3). Heat is then added to the air until a final state of
177\\\\deg Cand
620kPais reached. Calculate the change in entropy during the process using\ a) Approximate analysis (constant specific heats)\ b) Exact analysis (variable specific heats)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


