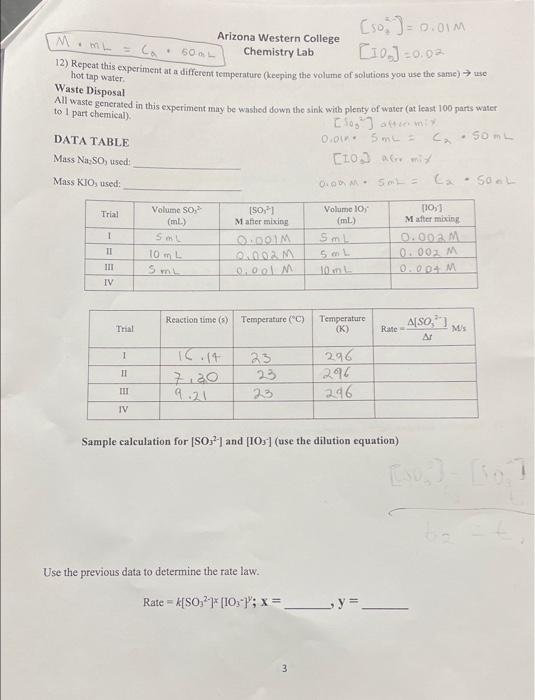
Procedure 1) You'll need to make solutions of 0.01 M sodium sulfite (50 mL) and 0.02 M potassium iodate (50 mL). The sodium sulfite solution requires H2SO (0.25 mL) to help facilitate the reaction. 2) There will be four trials. Each trial will have a total volume of 50.0 ml (see instruction #6). 3) You will decide the volumes, and thus the concentrations, of sodium sulfite and potassium iodate to use in the experiment. Volumes between 5 and 15 mL work best. It is suggested that for one trial you use 5.0 mL of the sulfite solution and 5.0 mL of the iodate solution. 4) Vary the concentrations of so-and 10s independently (i.e., change only one variable at a time). 5) Have your instructor check the planned procedure before you begin the experiment. 6) Place a small magnetic stir bar in a 125 ml Florence flask and add the following: a. X mL 0.01 M Na2SO3 b. 1 ml 1% starch solution c. Y mL 0.02 M KIO, you'll use d. 50- (X+Y+1) mL DI water 7) Measure the temperature of your solution using a thermometer 8) Set the flask with Na2SO3 and starch on a stirrer (these are in AS 211) and begin stirring at a moderate rate. 9) Measure Y mL of 0.02M KIO, into a beaker. 10) Coordinate this with your partner before you begin. Start the reaction by adding your chosen quantity of IO, then immediately start the stopwatch. Important: you will start the stopwatch at the same instant that the 103 solution begins to mix with the other solution. 11) The endpoint of the reaction is the point at which the solution turns blue-black. Stop the stopwatch the moment the blue-black color appears. Record the time in the data table in the data table. M. . 50 hot tap water Waste Disposal [soi] = 0.01M Arizona Western College Chemistry Lab [10] -0,02 [ 12) Repeat this experiment at a different temperature (keeping the volume of solutions you use the same) use to part All waste generated in this experiment may be washed down the sink with plenty of water (at least 100 parts water Choo.com DATA TABLE 0.01. SmL = . Som Mass Na SO, used (10) AG mi O. SE SOL Mass KIOs used: Trial Volume 10 (mL) 1 Volume SO, (ml) SI 10 mL (S0,1 Mafter mixing 0.001M 01002 M 0.00 M Sml 10,1 Matter mixing 0.002 M 0.002 M 0.00 M SmL 10 m IIT Smy IV Reaction time (s) Temperature (C) Temperature (K) Also Trial Rate MS Ar 1 TI 7,20 9.21 23 23 23 296 296 296 III IV Sample calculation for (SO2] and [105] (use the dilution equation) Use the previous data to determine the rate law. Rate = k[50,27 [10:-}; x= _y= 3 Arizona Western College Chemistry Lab Solve your rate law for k and fill in the table below. Show your work on the side. Rate Constant Trial 1 II III IV Practice Questions: 1) Use the Arrhenius equation to determine the activation energy, E., for this reaction 2) What effect does changing the concentration of 10, and So, have on the rate of reaction? Be specific. Arizona Western College Chemistry Lab 3) Predict the rate of the reaction if you had a starting (SO )= 0.15M and [IO;") 0.080M. 4) Explain the purpose of adding starch to the reaction. 5) Predict what would happen if Na2SO, did not react with the produced 13. Procedure 1) You'll need to make solutions of 0.01 M sodium sulfite (50 mL) and 0.02 M potassium iodate (50 mL). The sodium sulfite solution requires H2SO (0.25 mL) to help facilitate the reaction. 2) There will be four trials. Each trial will have a total volume of 50.0 ml (see instruction #6). 3) You will decide the volumes, and thus the concentrations, of sodium sulfite and potassium iodate to use in the experiment. Volumes between 5 and 15 mL work best. It is suggested that for one trial you use 5.0 mL of the sulfite solution and 5.0 mL of the iodate solution. 4) Vary the concentrations of so-and 10s independently (i.e., change only one variable at a time). 5) Have your instructor check the planned procedure before you begin the experiment. 6) Place a small magnetic stir bar in a 125 ml Florence flask and add the following: a. X mL 0.01 M Na2SO3 b. 1 ml 1% starch solution c. Y mL 0.02 M KIO, you'll use d. 50- (X+Y+1) mL DI water 7) Measure the temperature of your solution using a thermometer 8) Set the flask with Na2SO3 and starch on a stirrer (these are in AS 211) and begin stirring at a moderate rate. 9) Measure Y mL of 0.02M KIO, into a beaker. 10) Coordinate this with your partner before you begin. Start the reaction by adding your chosen quantity of IO, then immediately start the stopwatch. Important: you will start the stopwatch at the same instant that the 103 solution begins to mix with the other solution. 11) The endpoint of the reaction is the point at which the solution turns blue-black. Stop the stopwatch the moment the blue-black color appears. Record the time in the data table in the data table. M. . 50 hot tap water Waste Disposal [soi] = 0.01M Arizona Western College Chemistry Lab [10] -0,02 [ 12) Repeat this experiment at a different temperature (keeping the volume of solutions you use the same) use to part All waste generated in this experiment may be washed down the sink with plenty of water (at least 100 parts water Choo.com DATA TABLE 0.01. SmL = . Som Mass Na SO, used (10) AG mi O. SE SOL Mass KIOs used: Trial Volume 10 (mL) 1 Volume SO, (ml) SI 10 mL (S0,1 Mafter mixing 0.001M 01002 M 0.00 M Sml 10,1 Matter mixing 0.002 M 0.002 M 0.00 M SmL 10 m IIT Smy IV Reaction time (s) Temperature (C) Temperature (K) Also Trial Rate MS Ar 1 TI 7,20 9.21 23 23 23 296 296 296 III IV Sample calculation for (SO2] and [105] (use the dilution equation) Use the previous data to determine the rate law. Rate = k[50,27 [10:-}; x= _y= 3 Arizona Western College Chemistry Lab Solve your rate law for k and fill in the table below. Show your work on the side. Rate Constant Trial 1 II III IV Practice Questions: 1) Use the Arrhenius equation to determine the activation energy, E., for this reaction 2) What effect does changing the concentration of 10, and So, have on the rate of reaction? Be specific. Arizona Western College Chemistry Lab 3) Predict the rate of the reaction if you had a starting (SO )= 0.15M and [IO;") 0.080M. 4) Explain the purpose of adding starch to the reaction. 5) Predict what would happen if Na2SO, did not react with the produced 13