Question: Process Description The facility will use an integrated chlorination-oxychlorination process which proceeds in three stages: the chlorination of ethylene in the vapour phase to make
Process Description
The facility will use an integrated chlorination-oxychlorination process which proceeds in three stages: the chlorination of ethylene in the vapour phase to make ethylene dichloride (EDC); the thermal cracking of EDC to form VCM and hydrogen chloride; and the oxychlorination of ethylene with recycled hydrogen chloride to make more EDC. The overall process flow is shown schematically in Figure 1.
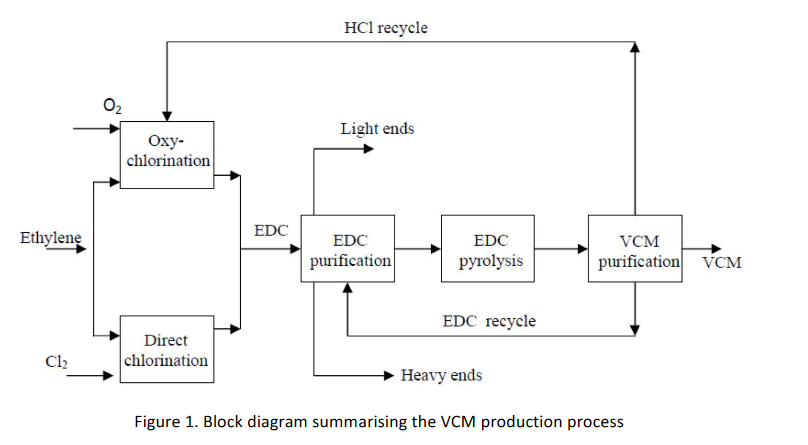
The main reactions are: Direct chlorination: C2H4 + Cl2 C2H4Cl2 (H = -180 kJ/mol) Oxychlorination: C24 + 2 Cl + 2 C24Cl2 + 2 ( = -239 kJ/mol) EDC pyrolysis: C2H4Cl2 C2H3Cl + HCl (H = 71 kJ/mol) Overall reaction 2C2H4 +Cl2 +O2 2C2H3Cl + H2O
The capacity of the plant will be 2.2 million te/yr of VCM. All feedstock will be fed via below ground pipelines from an adjacent chemical plant. Chlorine will be supplied to the site as a pressurized liquid via a 150 mm diameter pipeline. It will initially be fed into 2 50 te capacity buffer horizontal pressure vessels (3m diameter 15m length), operating at ambient temperature. The two buffer storage vessels are operated in parallel (inlet lines for the two vessels are manifolded together and outlet lines for the two vessels are manifolded together). Liquid chlorine will be pumped from the buffer storage through a vapouriser (located within the process area) which raises the liquid to a temperature of 30C and the vapour (at 8.7 barg) is fed under pressure control to the direct chlorination reactor. Ethylene and oxygen are supplied to the site as gas into buffer storage vessels under pressure control maintaining a pressure of 70 barg in the ethylene buffer vessels and 40 barg in the oxygen buffer vessels. Buffer storage for each consists of 4 horizontal pressure vessels 3m diameter and 15m long. All the oxygen buffer storage vessels are operated in parallel (all inlet lines are manifolded together, and all outlet lines are manifolded together which feed the oxychlorination reactor). The ethylene buffer storage vessels are operated in 2 parallel pairs (all inlet lines are manifolded together but outlets are manifold together separately for each pair - one pair to feed the direct chlorination reactor and the other pair to feed the oxychlorination reactor). Ethylene and oxygen are fed to the reactors by flow ratio control (ratio with chlorine for the direct chlorination and with hydrogen chloride for the oxychlorination reactor).
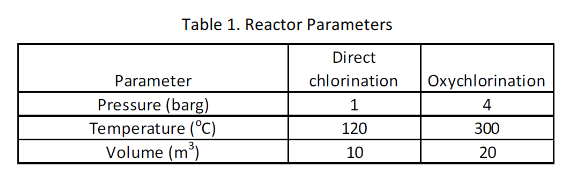
The HCl recycle line consists of a 150 mm diameter line, 100 m in length and operates at a pressure of 50 barg and a temperature of 40C which feeds the oxychlorination reactor under pressure control. The parameters associated with the two reactors are as follows:
The VCM product will be sent as a pressurized liquefied gas to product buffer storage which consists of 4 50 te horizontal pressure vessels (3 m diameter 15 m length) at ambient temperature. These vessels are operated in 2 parallel pairs (all inlet lines are manifolded together but outlets are manifold together separately for each pair). From each pair of buffer vessels the product is transferred under level control to 2 separate 150 mm diameter below ground pipelines which provide feedstock for an adjacent site for the manufacture of PVC. The ethylene and chlorine pipework from the buffer storage area to the process area is all 150 mm in diameter and for ethylene totals 300 m in length and 260 m in length for chlorine. The pipework from the process area to the VCM buffer storage is 150 mm and totals 170 m in length. The site will be fitted throughout with an effective fire and gas detection system, so that any loss of containment can be assumed to be rapidly detected. Isolation is to be provided at the inlets and outlets of all the buffer storage vessels, both reactors and the chlorine vaporizer. In the initial design isolation will be by manual valves, which can take up to 15 minutes in the event of a major event. Your input will be required in identifying if remote/automated isolation activation are required to reduce the consequences of potential major accidents.
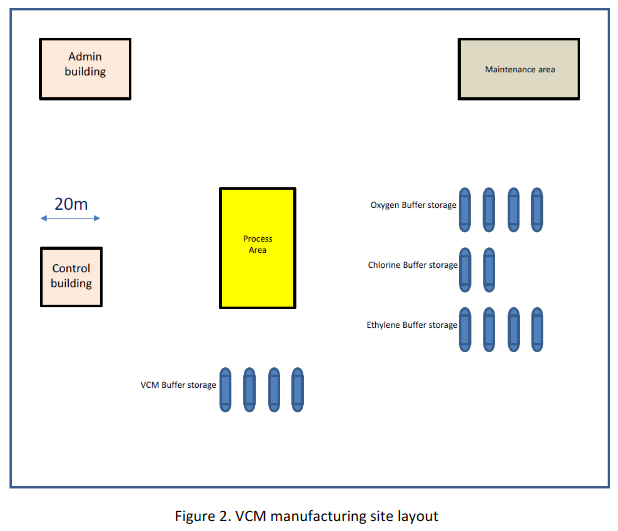
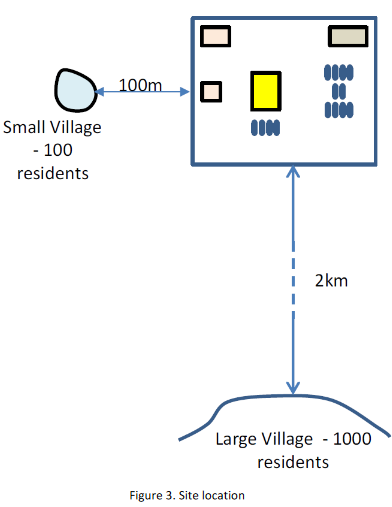
The requirements for active and passive fire protection have not yet been specified, and again your input will be required to suggest what measures might be the most effective in reducing the consequences of potential major accidents. On site fire response capability will be very limited with reliance to be placed on the external fire service and mutual aid from the neighbouring sites. All of the outdoor operators have been trained as first responders. However, response equipment is limited to hose reels located around the site connecting to a 100 mm diameter fire ring main operating at 15 bar. A control building will house 20 people. The intention is for this building to be protected against fire, blast and gas ingress although the design has not yet been fully developed. There will also be an administration building on site for 50 people. This will be a two-storey concrete framed building and will not be specifically designed as a fire and blast resistant building and will be naturally ventilated. An outdoor space has been allocated for maintenance work where typically there will be 5 workers present. The VCM process area is congested and equates to a congested volume of 12,000 m3. The rest of the site is uncongested. At a distance of 100 m to the West of the site is located a small village of 150 people. A second, larger village of 1500 people is located 2 km to the South of the site. For both villages assume that 50% of the population are outdoors and 50% are indoors in conventional brick houses. The layout and location are shown in Figures 2 and 3.
QUESTIONS
Q1
a) Discuss, briefly, the pros and cons of using simple consequence models of the type introduced in the module. If the company had a limited budget for more detailed simulations, how would you prioritise which scenarios to investigate?
b) List all the types of major accident events which can occur from pipework leaks in each area on the site (e.g. ethylene gas jet fire in process area). You may ignore any failures associated with the import and export pipelines (which are all below ground). For the process area, you need only consider feed/recycle lines associated with the reactors. For this exercise, VCM acute toxic effects can be discounted - you need only consider flammable hazards for this material. You need not consider any events associated with oxygen.
c) For events listed under Q1a) Determine the maximum number of onsite and offsite fatalities. '' You should perform hand calculations, showing all workings and stating any assumptions''
d) Determine which of the initial events can lead to a second event which has the potential for consequences which are more severe than the initial event (escalation). Describe how the escalation occurs (e.g. due to VCE in process area) and list all of the resulting escalation events (e.g. chlorine toxic release from liquid pipe rupture in chlorine buffer storage area).
e) The possibility of escalation from leaks in either the VCM or ethylene buffer storage is of particular concern. For each of the escalation events as identified under Q1c) due to leaks in these areas, determine the maximum number of offsite (public) fatalities.
Assumptions to be Used in Calculations
Calculations
Leaks
For leaks, you can assume that a 50 mm diameter hole is a representative event. When determining release rates for liquefied gas releases the formula for a liquid release ignoring the effect of liquid head may be used. A discharge coefficient of 0.6 can be applied in release calculations. It is assumed that the inventory of HCl is contained wholly within the recycle line and any backflow of HCl from the rest of the process can be ignored.
Dispersion
For flashing liquid releases, you should assume that all of the release enters the vapor cloud. For all calculations an ambient temperature of 15C, a windspeed of 5 m/s and a neutral (C) atmospheric stability should be assumed. When calculating atmospheric dispersion, the dispersion coefficients (for C Stability) may be calculated by the following formulae:

Jet Fires
For jet fires, only direct flame engulfment need be considered. You should assume that people indoors are unprotected from direct flame engulfment. In the event of direct flame engulfment it can be assumed that there are 100% fatalities indoors (unless the building is specifically designed to withstand this event) and 100% fatalities outdoors.
Flash Fires and VCEs
For flash fires you can assume that there are 100% fatalities within the flammable cloud (determined by the concentration contour to the Lower Flammable Limit) for outdoor populations and 50% for indoor populations for naturally ventilated buildings. For VCEs, assume that the whole of a congested volume is filled with a stoichiometric mixture of ethylene. Toxicity For toxic releases, assume exposure time is limited to release duration. For toxic clouds assume that indoor fatality rate equals half of the corresponding outdoor fatality rate. All releases should be assumed to be in the centre of the relevant area.
For toxicity calculations the following Probit constants should be used:
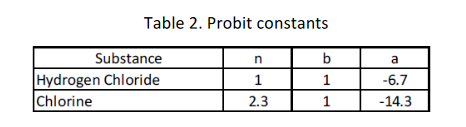
where time is in minutes and concentration is in mg/m3
Blast Overpressure
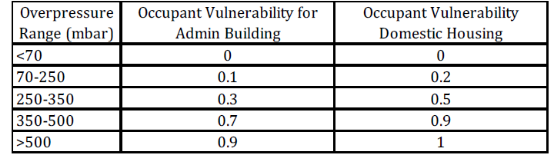
The following vulnerabilities to blast overpressure apply to indoor populations:
Table 3. Building occupant vulnerability to blast overpressure
Hazard Zones
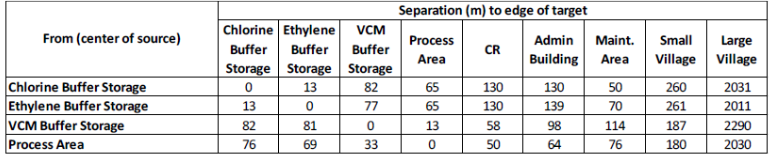
Assume that if a hazard zone reaches the edge of a population the whole of that population will experience the same fatality rates (separately for indoor and outdoor populations). Where the consequences are dependent on the direction of an event, four possible directions event to North, East, South and West should be considered. Any population within range and within the relevant sector (within 45 either side of the direction) can be exposed to the event. Separation distances are provided below: Table 4. Separation distances (centre of source to edge of target)
Escalation
Pipework and vessel rupture is possible if flame engulfment exceeds 15 minutes. Pipework rupture is possible if blast overpressure exceeds 200mbar. The possibility of vessel rupture due to blast overpressure can be ignored. For each area, escalation should be assumed if these thresholds are exceeded at any point in the respective area. Vessel failures in the process area can be discounted (vessel inventories are small in this area).
For rupture of a vessel containing a toxic material, it can be assumed that the contents are released over a period of 60 seconds leading to an exposure time of 5 minutes
Figure 1. Block diagram summarising the VCM production process Table 1. Reactor Parameters Figure 2. VCM manufacturing site layout Figure 3. Site location y=104x0.984z=61x0.911 where y and z are in m and x is downwind distance in km. All releases should be treated as continuous (you may wish to comment on this...) Table 2. Probit constants \begin{tabular}{|l|c|c|} \hline OverpressureRange(mbar) & OccupantVulnerabilityforAdminBuilding & OccupantVulnerabilityDomesticHousing \\ \hline500 & 0.9 & 1 \\ \hline \end{tabular} \begin{tabular}{|l|c|c|c|c|c|c|c|c|c|} \hline \multicolumn{1}{|c|}{ From (center of source) } & \multicolumn{9}{|c|}{ Separation (m) to edge of target } \\ \cline { 2 - 9 } & ChlorineBufferStorage & EthyleneBufferStorage & VCMBufferStorage & ProcessArea & CR & AdminBuilding & Maint.Area & SmallVillage & LargeVillage \\ \hline Chlorine Buffer Storage & 0 & 13 & 82 & 65 & 130 & 130 & 50 & 260 & 2031 \\ \hline Ethylene Buffer Storage & 13 & 0 & 77 & 65 & 130 & 139 & 70 & 261 & 2011 \\ \hline VCM Buffer Storage & 82 & 81 & 0 & 13 & 58 & 98 & 114 & 187 & 2290 \\ \hline Process Area & 76 & 69 & 33 & 0 & 50 & 64 & 76 & 180 & 2030 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


