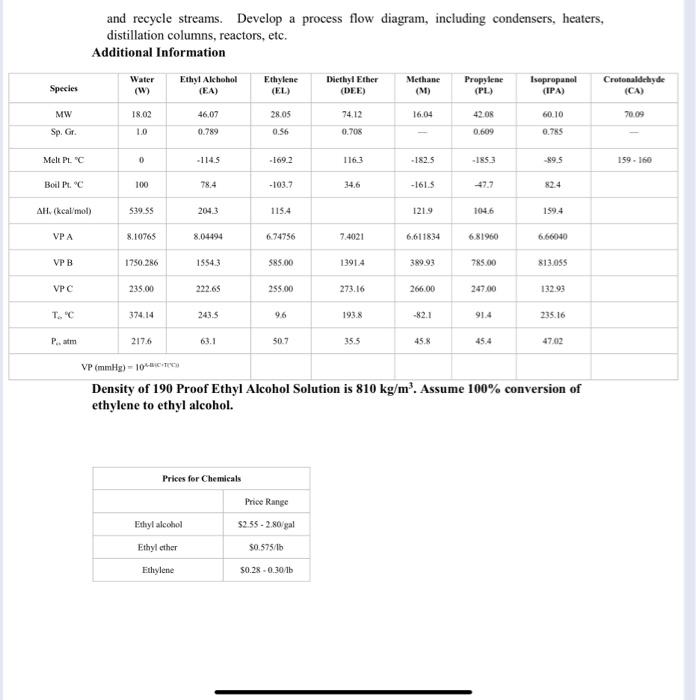
process design problem

Problem: To utilize an excess of approximately 75 x 10 kg/yr of ethylene the Gulf Coast plant is producing by manufacturing ethyl alcohol. The sales department indicates 150,000 m/yr of 190 percent ethyl alcohol can be sold. Design Team Assignment: Investigate the design of a plant to convert substantial part of the excess ethylene to 150,000 m /yr of 190 proof ethanol (85.44 mole % ethyl alcohol and 14.56 mole % water). Ethylene Feed: 96 mole % ethylene 3 mole propylene 1 mole % methane Assume 100% Conversion Ethylene (CHA) + Water (H20) - Ethanol (CHO) Main Reaction: 2 Ethanol (CHO) - Diethyl Ether (C:H).0) Propylene (CH) + Water (HO) - Isopropanol (CHO) Methane (CH) - Coking Crotonaldehyde (C.H.O) (Waste) Feed Composition: 4:1 Water to Ethylene ratio for complete conversion 1. Maximum Potential Profit - The manufacturer price of the ethylene feed is $ 0,18/1b. Given the information provided below, calculate maximum profit range per year (Not including equipment and operating costs). 2. Draw schematic of overall boundary (showing input and outputs) of the ethyl alcohol process. Develop a box flow diagram, including reactor, mixing and splitting points, separators, purge and recycle streams. Develop a process flow diagram, including condensers, heaters, distillation columns, reactors, etc. Additional Information Species Water (W) Ethyl Alchohol (EA) Ethylene (EL) Diethyl Ether (DEE) Methane (M) Propylene (PL) Isopropanol (IPA) Crotonaldehyde (CA) MW 18.02 46,07 28.05 16.04 42.08 60.10 70.09 74.12 0.705 Sp. Gr. 1.0 0.789 0.56 0.609 0.785 Melt PLC 0 -1145 -169.2 -182.5 -1853 89.5 159 160 Boil Pt." 100 78.4 -103.7 34,6 -161.5 47,7 AH, (kcal/mol) 539.55 204,3 115.4 1219 104.6 159.4 VPA 8.10765 8.04494 6.74756 7.4021 6,611834 6.81960 6.66040 VPB 1750.286 15543 585.00 13914 38993 785.00 813.055 VPC 235.00 222.65 255.00 273.16 266.00 247.00 132.93 T.C. 374.14 243.5 96 193.8 -82.1 91.4 235.16 Pam 217.6 63.1 50.7 355 45. 45.4 47.02 VP (mmHg) - 106CT Density of 190 Proof Ethyl Alcohol Solution is 810 kg/m. Assume 100% conversion of ethylene to ethyl alcohol. Prices for Chemicals Price Range Ethyl alcohol $2.55 - 2.80/gal Ethyl ether $0.575/16 Ethylene $0.28-0.30/1b Problem: To utilize an excess of approximately 75 x 10 kg/yr of ethylene the Gulf Coast plant is producing by manufacturing ethyl alcohol. The sales department indicates 150,000 m/yr of 190 percent ethyl alcohol can be sold. Design Team Assignment: Investigate the design of a plant to convert substantial part of the excess ethylene to 150,000 m /yr of 190 proof ethanol (85.44 mole % ethyl alcohol and 14.56 mole % water). Ethylene Feed: 96 mole % ethylene 3 mole propylene 1 mole % methane Assume 100% Conversion Ethylene (CHA) + Water (H20) - Ethanol (CHO) Main Reaction: 2 Ethanol (CHO) - Diethyl Ether (C:H).0) Propylene (CH) + Water (HO) - Isopropanol (CHO) Methane (CH) - Coking Crotonaldehyde (C.H.O) (Waste) Feed Composition: 4:1 Water to Ethylene ratio for complete conversion 1. Maximum Potential Profit - The manufacturer price of the ethylene feed is $ 0,18/1b. Given the information provided below, calculate maximum profit range per year (Not including equipment and operating costs). 2. Draw schematic of overall boundary (showing input and outputs) of the ethyl alcohol process. Develop a box flow diagram, including reactor, mixing and splitting points, separators, purge and recycle streams. Develop a process flow diagram, including condensers, heaters, distillation columns, reactors, etc. Additional Information Species Water (W) Ethyl Alchohol (EA) Ethylene (EL) Diethyl Ether (DEE) Methane (M) Propylene (PL) Isopropanol (IPA) Crotonaldehyde (CA) MW 18.02 46,07 28.05 16.04 42.08 60.10 70.09 74.12 0.705 Sp. Gr. 1.0 0.789 0.56 0.609 0.785 Melt PLC 0 -1145 -169.2 -182.5 -1853 89.5 159 160 Boil Pt." 100 78.4 -103.7 34,6 -161.5 47,7 AH, (kcal/mol) 539.55 204,3 115.4 1219 104.6 159.4 VPA 8.10765 8.04494 6.74756 7.4021 6,611834 6.81960 6.66040 VPB 1750.286 15543 585.00 13914 38993 785.00 813.055 VPC 235.00 222.65 255.00 273.16 266.00 247.00 132.93 T.C. 374.14 243.5 96 193.8 -82.1 91.4 235.16 Pam 217.6 63.1 50.7 355 45. 45.4 47.02 VP (mmHg) - 106CT Density of 190 Proof Ethyl Alcohol Solution is 810 kg/m. Assume 100% conversion of ethylene to ethyl alcohol. Prices for Chemicals Price Range Ethyl alcohol $2.55 - 2.80/gal Ethyl ether $0.575/16 Ethylene $0.28-0.30/1b